Africa Reach Regulation for Various Sectors
The Department of Agriculture, Forestry and Fisheries (DAFF) is responsible for the registration of pesticides in South Africa. Pesticides are regulated under the Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act, 1947 (Act No. 36 of 1947). In June 2019, after the reconfiguration of government departments, the agriculture function of DAFF was substituted by the Department of Agriculture, Land Reform and Rural Development.
Fertilizers and Farm Feeds Parliament approved the Agricultural Remedies and Stock Remedies Act in 1947, which regulates pesticides and their use, among other things. The Act has been changed several times since then, but it has never been comprehensively overhauled. Since the Act's establishment, the operations of the Department of Agriculture, Forestry, and Fisheries (DAFF), the entity that administers it, have not been subjected to public scrutiny. Furthermore, many of the over 3000 pesticides licensed for use in South Africa have not been re-evaluated in years.
The objectives of this Act are:
- To improve the legislative framework and ensure that South Africans are better protected from health and environmental risks posed by pesticides.
- To encourage the development and use of alternative products and techniques and reduce dependence on chemical plant protection products.
- To integrate relevant international agreements and initiatives from other government departments.
- Increased transparency, access to information and improved public participation in the registration of pesticides.
To register a pesticide product in South Africa, the applicant company must have a local legal entity to represent them.
A legal entity might be a person or a firm registered with the Department of Trade and Industry under the Company Act.
Pesticides are only allowed to be registered if they are approved to be effective, safe, and of high quality.
For oversea manufacturers, they should appoint an Authorized Representative (AR).
Registration consists of the following steps:
- Five batch analytical reports are used to verify chemical and toxicological equivalency.
- Data from a GLP (Good Laboratory Practice) approved independent laboratory is required.
- Details about the production.
- At least three biological efficacy data reports on local cockroach species have been completed. The South African Bureau of Standards (SABS) conducts such research in South Africa.
- The active ingredients and the formed product's chemical-physical data must also be submitted. All product specifications, such as data on storage stability, must be submitted.
- Details about how the product works, including the method of action.
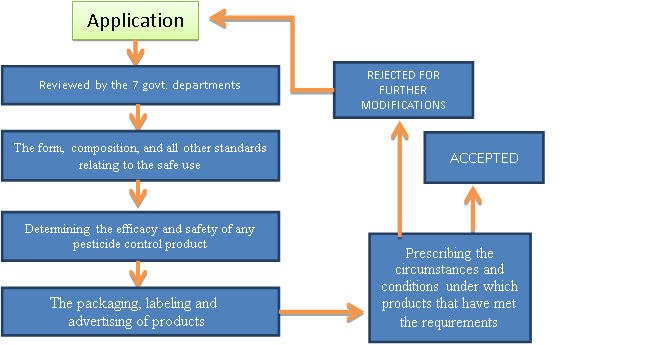
The Department of Agriculture, Forestry, and Fisheries (DAFF) is responsible for ensuring that pesticides are appropriately labelled and that all information necessary for safe use is prominently displayed. Information should be given in such a way that a person can grasp the risks and acquire a sense of proportion to make an informed decision about whether those risks are acceptable. The labelling policy would be in line with the new Globally Harmonized System (GHS) of chemical classification and labelling.
The words "POISON EXTREMELY TOXIC" must appear in red letters no smaller than half the size of the product's name on a contracting background on the main panel of the label and the skull and crossbones; the second group requires the word "POISONOUS," and the third group requires the word "CAUTION" in a similar print. The least dangerous group does not require any toxicity information, but any indication that a product is possibly safe is permitted on the label or in promotional materials.
Toxic pesticide labelling is also subject to the regulations outlined in health law.
GPC can help you with:
- Identification of compliance requirements under various guidelines including all data requirements.
- Data gap analysis and pre-assessment support
- Technical documentation support
- Pre and post-submission support and technical liaison with authorities.
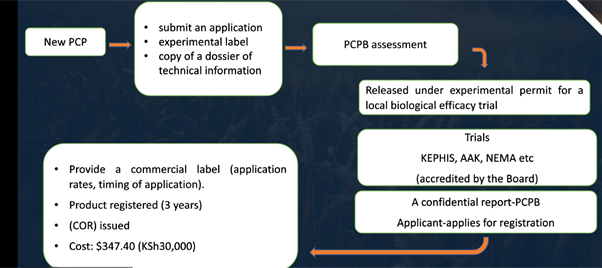
In Kenya, Pest Control Products Board (PCPB) is the pesticide management department, responsible for pesticide registration, controlling and managing pesticide, importing/ exporting, production, distribution, and application. The main pesticide regulation is the Pest Control Products (PCP) Act, Cap 346. Important Mandate of PCPB “Assessing the safety, efficacy, quality, merit and economic value of PCP with a view to approving them, if found suitable in local situation”.
All applications for consideration of approval of PCP must be accompanied by submission of risk assessment reports (2019/2021- Risk assessment workshops).
Risk assessment report needs to be submitted:
(1) During submission of summary dossier for new products.
(2) Upon completion of efficacy trials for label extension. Following conclusion of the local experimental efficacy trials.
Applicant will be required to provide detailed human (occupational and dietary) and environmental (Bees) risk assessment reports for all applications of new products and label extensions to ensure any PCP do not pose unacceptable risks (July 2021).
Applicants needs to provide the summary of risk assessment:
-
Quality of the data referenced for hazard assessment (e.g., Experimental design and quality of the critical study or studies).
-
Kenya Good agricultural practice (GAP) Table for efficacy and residue
-
Operator/Worker/Consumer exposure models and local risk assessment.
-
Bee exposure model and the local situation
-
The Input and output page of the exposure should be attached
-
Brief discussion of conclusion arising from the model’s output
-
Discussion on the proposed realistic pesticide risk management
PCPB controls the importation of pesticides through various registrations and licensing:
-
Agency licensing- pesticides manufacturers outside Kenya (validity, 1 year)
-
Product registration- pesticide products imported to Kenya (validity 3 years)
-
Premise licensing- premises handling pesticides (validity, 1 year)
-
Import permit
-
Import standardization mark (ISM)
-
Environmental protection
During the licence application process of importation or exportation of a pest control product for commercial purposes, applicants should specify its purpose such as “for resale”, “for manufacturing purpose” or “for importer’s own use”, etc.
-
Introductory fee of KS 10,000 to obtain an experimental permit.
-
A certificate of registration will cost the applicant $347.40 (KSh30, 000)
-
While a renewal certificate of registration not exceeding two years costs $231.60 (KSh20, 000)
-
Identification of compliance requirements under various guidelines including all data requirements.
-
Data gap analysis and pre-assessment support
-
Technical documentation support
-
Pre and post submission support and technical liaison with authorities.
There is no national inventory in South Africa at the moment. However, under the Hazardous Substances Act (Act No.15 of 1973), there is a chemical list concerning hazardous substances. The hazardous substances are divided into 4 groups.
-
Group I: Industrial chemicals (IA) and pesticides (IB)
-
Group II: 9 classes of wastes* excluding Class 1 (Explosives) and Class 7 (Radioactive substances)
-
Group III: Electronic products
-
Group IV: Radioactive substances
Substances or mixtures can be included to the list after the Ministry publishes a notice in the Gazette and after a public consultation. A period of not less than three months is required for adding substances to the list.
The list of group IA hazardous substances is listed below. Mixtures of the listed hazardous substances are also considered hazardous:
-
Aluminium phosphide
-
Arsenic and its salts
-
Antimony potassium tartrate
-
Antimony sodium tartrate
-
Barium and its salts except barium sulphate
-
Cantharidin
-
Cyanides of potassium and sodium
-
Other poisonous cyanide substances, preparations and admixtures containing or yielding the equivalent of one-tenth per cent or more of hydrocyanic acid
-
Fluoroacetic acid (mono), its salts and derivatives
-
Hydrocyanic acid
-
Lead acetate
-
Mercuric ammonium chloride
-
Phosphorus, yellow
-
Strychnine
-
Thallium
-
Zinc phosphide
-
Carbon tetrachloride (added by Government Notice R1705 of 1995)
-
Leaded paint (added by Government Notice R801 of 2009)
* The 9 Classes are: Class 1 (Explosives), Class 2 (Gases), Glass 3 (Flammable Liquids), Class 4 (Flammable solids), Class 5 (oxidizing substances and organic peroxides), Class 6 (Toxic and infectious substances), Class 7 (Radioactive substances), Class 8 (Corrosives), Class 9 (Other miscellaneous substances).
Chemicals in South Africa are currently regulated by several regulations. The major legal frameworks for regulating chemicals are the Hazardous Substances Act, 1973 (Act No.15 of 1973) and the Occupational Health and Safety Act (85 Of 1993). Certain chemicals are also regulated by the National Environmental Management Act, 1998 (107 Of 1998).
Hazardous Substances Act, 1973 (Act No.15 of 1973)
The Hazardous Substances Act which is authorized by the Department of Health (DOH), provides for the control of substances, which may cause injury or ill health to, or death of human beings. The Act sets requirements on the prohibition and control of the importation, manufacture, sale, use, operation, application, modification, disposal, or dumping of hazardous substances. The Act is administered by the Minister of Health, who exercises control over the various products by declaring them to be in any one of four specified groups of hazardous substances. Once a group is declared, the Minister can promulgate regulations controlling various aspects of their handling.
Occupational Health and Safety Act No.85 of 1993
The Occupational Health and Safety Act of 1993 is South Africa’s principal legislation
concerning health and safety of employees. The Act places the responsibility on the employer to ensure a safe and healthy working environment. It is administered by the Department of Labour (DOL) directly under the control of the Chief Directorate of Occupational Health and Safety.
The Act covers all substances and materials manufactured and stored in the workplace including:
-
-
Pesticides (agricultural, public health and consumer use)
-
Fertilizers
-
Industrial chemicals (used in manufacturing/processing facilities)
-
Petroleum products
-
Consumer chemicals
-
Chemical wastes (the manufacture and handling only)
-
National Environmental Management Act, 1998 (Act No.107 of 1998)
The National Environmental Management Act authorizes the Department of Environmental Affairs (DEA) to prohibit or control certain substances or chemicals that pose threat to human health and the environment. So far only the following two subsidiary regulations have been implemented under the Act:
-
GN R341: Regulations for the Prohibition of use, manufacturing, import and export of asbestos and asbestos containing materials (2008).
-
GN R549: Regulations to phase out the use of Polychlorinated Biphenyls (PCBs) materials and Polychlorinated Biphenyl (PCB) contaminated materials (2014)
The authorities in charge of chemical regulations in South Africa are:
-
Department of Health (DOH) - Hazardous Substances Act, 1973
-
Department of Labour (DOL) - Occupational Health and Safety Act (85 Of 1993)
-
Department of Environmental Affairs (DEA) - National Environmental Management Act, 1998 (107 Of 1998)
The Hazardous Chemical Substance Regulations under the Occupational Health and Safety Act require that manufacturers, importers, and suppliers of hazardous chemical substances for use at workplace provide free safety data sheets that are compliant with ISO 11014 or national standards. Non-South African manufactures must appoint a nominated registered agent located in South Africa.
Under the Hazardous Substances Act (1973), hazardous substances are divided into 4 groups. Group I and II consist of hazardous chemical substances and mixtures which might cause harm to human beings and are declared as such by notice in the Government Gazette. The list of Group I hazardous substances can be found in the Inventory Section. Group III consists of hazardous electronic products declared as such by notice in the Government Gazette. Group IV contains radioactive materials which the Minister has by notice in the Gazette declared to be a Group IV hazardous substance and which is used or intended to be used for medical, scientific, agricultural, commercial, or industrial purpose, and any radioactive waste arising from such radioactive material.
The classification of hazardous chemical agents is regulated by Regulation 14 of the Regulations for Hazardous Chemical Agents 2021. This regulation requires manufacturers and importers of chemical agents to do the following before the chemical agents are supplied to a workplace:
-
Determine whether the chemical agent is a Hazardous Chemical Agent (HCA) by carrying out a hazard assessment, with reference to the cut-off values provided Annexure 1 of Regulation 14.
-
If the substance, mixture, or article is an HCA, ensure that a GHS classification is carried out for the HCA.
-
Review the GHS classification if a change is made in the composition of the HCA.
The Hazardous Substances Act specifies that Group I substances can only be sold by someone who has a license from the Department of Health. The Group I Hazardous Substances Regulations regulates the management of the relevant chemicals in terms of:
-
Licensing procedures
-
Persons to whom a licence may be granted
-
Conditions of sale or supply
-
Records to be kept
-
Prohibition of sale to persons under 16 years of age
-
Labelling
-
Duties of inspectors and analysts
-
Disposal of empty containers
-
Fines
No licensing requirements or other control measures exist in respect of Group II
hazardous substances. For Group IV Hazardous Substances (Radioactive Materials) written authority from the Director-General is required to produce or otherwise acquire, dispose of, import or export, be in possession of, use, convey or cause to be conveyed.
The manufacturers, importers, and retailers are the main actors in the process for registration. South African manufacturers can apply directly while overseas manufacturers must apply through a South Africa based legal entity. Following documents have to be submitted for registration:
-
A copy of their VAT certificate or
-
a certified copy of their identity document if they are not in possession of a VAT certificate.
-
Recommendation from South African Police Service (SAPS) in the event of chemicals listed in the 1988 Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances being imported.
-
Recommendation from the Department of Environmental Affairs if the chemicals are listed in the Montreal Protocol on Substances that Deplete the Ozone Layer.
-
After submission It may take up to three days for an import permit to be granted.
-
International Trade Administration Commission of South Africa (ITAC) does not charge any service fees, but importers will have to pay customs duty at South African Revenue Service (SARS). Customs duty is calculated as a percentage of the value of the goods (set in the schedules to the Customs and Excise Act).
Any person who contravenes one or more of the regulations in the Regulations for Hazardous Chemical Agents 2021 shall be guilty of an offence and liable to conviction to a fine or imprisonment for a period not exceeding six months. The amount of the fine is not disclosed. In the case of a continuous offence the penalty will be an additional fine of 500 ZAR (approximately US$ 27) or one day of imprisonment for each day on which the offence continues.

 Twitter
Twitter
