China Reach Regulation for Various Sectors
The Measures for the Registration of Environmental Management of New Chemical Substances were approved by the Ministry of Ecological Environment on February 17, 2020 and are hereby promulgated, effective January 1, 2021. The Measures for the Environmental Management of New ChemicalSubstances (Decree No. 7 of the Ministry of Environmental Protection) issued by the former Ministry of Environmental Protection on 19 January 2010 were repealed at the same time.
Article 1 These Measures are formulated in accordance with relevant laws and regulations and the Decision of the State Council on the Establishment of Administrative Permits for Administrative Approval Projects that are necessary to retain new chemical sishists, scientifically and effectively assess and control the environmental risks of new chemical substances, focus on new chemical substances that may pose greater risks to the environment and health, protect the ecological environment and safeguard public health.
Article 2 These Measures shall apply to the registration of environmental management for the research, production, import and processing of new chemical substances within the territory of the People's Republic of China, except for new chemical substances stored in the special customs area after import and exported in their entirety without any processing.
The following products or substances do not apply to these Measures:
(1) medicines, pesticides, veterinary drugs, cosmetics, food, food additives, feed, feed additives, fertilizers and other products, except for new chemical substances that are changed for other industrial uses, as well as raw materials and intermediates of the above-mentioned products;
(2) Radioactive substances.
Article 3 The term "new chemical substances" as mentioned in these Measures refers to chemical substances that are not included in the List of Existing Chemical Substances in China.
Chemical substances that have been included in the List of Existing Chemicals in China shall be environmentally called for environmental management according to existing chemical substances, but those chemical substances that are provided for in the List of Existing Chemicals in China for the implementation of environmental management for new uses shall be environmentally managed in accordance with new chemicals for industrial purposes other than those permitted.
The List of Existing Chemical Substances in China shall be formulated, adjusted and published by the competent department of ecological environment under the State Council, including chemical substances that have been produced, sold, processed or imported in the territory of the People's Republic of China before October 15, 2003, and chemical substances included after October 15, 2003 in accordance with the relevant regulations on environmental management of new chemical substances.
Article 4 The State shall implement a system of environmental management and registration of new chemical substances.
The registration of environmental management of new chemical substances is divided into routine registration, simple registration and filing. The producer or importer of a new chemical substance shall obtain a routine registration certificate for environmental management of new chemical substances or a simple registration certificate (collectively, a registration certificate) before production or before importing it, or handle the record of environmental management of new chemical substances.
Article 5 The registration of environmental management of new chemical substances shall follow the principles of science, efficiency, openness, fairness, fairness and convenience, adhere to source access, risk prevention and classification management, and focus on the control of new chemical substances that are persistent, bioaccumulative, harmful to the environment or health, or may exist in the environment for a long time and may pose greater risks to the environment and health.
Article 6 The competent department of ecological environment under the State Council shall be responsible for organizing and carrying out the national environmental management registration of new chemical substances, formulating supporting documents such as policies, technical norms and guidelines for the registration of new chemical substances, as well as the rules for registration and evaluation, and strengthening the construction of the registration and informationization of new chemical substances.
The competent department of ecological environment under the State Council shall organize the establishment of an expert committee on environmental risk assessment of chemical substances (hereinafter referred to as the committee of experts). The expert committee is composed of experts in chemistry, chemical, health, environment, economy, etc., and provides technical support for the registration and evaluation of environmental management of new chemical substances.
The competent local ecological environment departmentats at or above the municipal level of the district shall be responsible for the environmental supervision and management of the implementation of these Measures by the relevant enterprises and institutions that study, produce, import and process the use of new chemical substances in their respective administrative areas.
The technical institution sundered by the competent department of ecological environment under the State Council shall participate in the registration and evaluation of the environmental management of new chemical substances and undertake the specific work of the registration of new chemical environmental management.
Article 7 Enterprises and institutions engaged in the research, production, import and processing of new chemical substances shall abide by the provisions of these Measures, take effective measures to prevent and control the environmental risks of new chemical substances, and shall be responsible for the damage caused by them.
Article 8 The State encourages and supports the scientific research and application of environmental risk assessment and control technologies for new chemical substances, and encourages the research and application of environmentally friendly chemical substances and related technologies.
Article 9 All units and individuals shall have the right to report any violation of the provisions of these Measures to the competent department of ecological environment.
Article 10 Where the annual production of new chemical substances or the import volume of more than 10 tons, the general registration of environmental management of new chemical substances shall be conducted (hereinafter referred to as the regular registration).
If the annual production of new chemical substances or the import volume of more than 1 ton is less than 10 tons, the environmental management of the new chemical substances shall be subject to a simple registration (hereinafter referred to as the simple registration).
If one of the following conditions is met, the new chemical environmental management shall be filed (hereinafter referred to as the record):
(1) The annual production of new chemical substances or the import volume is less than 1 ton;
(2) A polymer with a content of no more than 2% of the new chemical substance monomer or reaction body or a polymer of low concern.
Article 11 An applicant for the registration of environmental management of new chemical substances shall, in accordance with the law within the territory of the People's Republic of China, be able to bear legal responsibility independently, and engage in the production or import ation of new chemical substances.
A production or trading enterprise that intends to export new chemical substances to the territory of the People's Republic of China may also act as an applicant, but shall designate enterprises and institutions registered in the territory of the People's Republic of China who can independently assume legal liability as agents, jointly fulfill their obligations for environmental management after registration and registration of new chemical substances, and assume responsibility in accordance with the law.
If the products of medicine, pesticides, veterinary drugs, cosmetics, food, food additives, feed, feed additives, fertilizers, etc. as stipulated in Article 2 of these Measures belong to new chemical substances and are to be changed for other industrial purposes, the producers, importers or processing users of the relevant products may be eligible as applicants.
Producers, importers or processing users of chemical substances that have been included in the List of Existing Chemicals in China and have implemented environmental management for new uses may be used for industrial purposes other than those permitted.
Article 12 Where an application is made for registration of new chemical environmental management, the applicant shall submit the registration application or filing materials to the competent department of ecological environment under the State Council and shall be responsible for the authenticity, completeness, accuracy and legality of the registration application or filing materials.
The State encourages applicants to share registration data on environmental management of new chemicals.
Article 13 If the applicant believes that the registration application or filing materials submitted by him involve trade secrets and requires information protection, it shall submit it at the time of application for registration or filing, and submit the materials on the necessity of applying for the protection of trade secrets. The competent department of ecological environment under the State Council may not protect trade secrets in accordance with the law for information that may have a significant impact on the environment and the public interest of health. The applicant may withdraw the request for information protection that has been made in writing.
The period of protection of marking information, such as the name of a new chemical substance, shall not exceed five years from the date of first registration or filing.
Staff members and relevant experts engaged in the registration of environmental management of new chemical substances shall not disclose trade secrets which should be protected according to law.
Article 13 If the applicant believes that the registration application or filing materials submitted by him involve trade secrets and requires information protection, it shall submit it at the time of application for registration or filing, and submit the materials on the necessity of applying for the protection of trade secrets. The competent department of ecological environment under the State Council may not protect trade secrets in accordance with the law for information that may have a significant impact on the environment and the public interest of health. The applicant may withdraw the request for information protection that has been made in writing.
The period of protection of marking information, such as the name of a new chemical substance, shall not exceed five years from the date of first registration or filing.
Staff members and relevant experts engaged in the registration of environmental management of new chemical substances shall not disclose trade secrets which should be protected according to law.
Article 14 The domestic testing institutions of the People's Republic of China that provide test data for the registration of new chemical substances for environmental management shall, in accordance with law, obtain the qualification of the inspection and testing institutions and carry out testing work in strict accordance with the relevant standards of chemical substance testing; The testing institution shall be responsible for the authenticity and reliability of the test results it has issued and shall be held responsible in accordance with the law.
The competent department of ecological environment under the State Council shall organize supervision and spot checks on the testing conditions and toxicology testing institutions.
The testing institutions outside the People's Republic of China that produce data on health toxicology or ecotoxicology testing shall comply with the internationally accepted requirements for good laboratory management.
Article 15 Where an application is made for routine registration, the applicant shall submit the following materials:
(1) The application form for regular registration;
(2) The physical and chemical properties of new chemical substances, health toxicology and ecological toxicology characteristics test report or data;
(iii) Environmental risk assessment reports on new chemical substances, including an assessment of the possible environmental risks of new chemicalsubstances to be applied for registration, the proposed environmental risk control measures and their appropriateness analysis, and the assessment of the existence of unreasonable environmental risks;
(4) A commitment letter to implement or transmit environmental risk control measures and environmental management requirements shall be signed by the legal representative of an enterprise or institution or its authorized person and sealed.
The relevant test reports and data as stipulated in ARTICLE 14 shall meet the needs of the environmental risk assessment of new chemical substances, and the ecotoxicology test report shall include test data completed using the test organisms of the People's Republic of China in accordance with the relevant standards.
For those belonging to highly hazardous chemical substances, the applicant shall also submit the social and economic benefits of the new chemical activities, including a description of whether the new chemical substance has a considerable or obvious advantage in the use of chemical substances in terms of performance and environmental friendliness, and fully demonstrate the necessity of the application activity.
In addition to the application materials set out in the preceding three paragraphs of this article, the applicant shall also submit other information on the environmental and health hazards of the new chemical substances he has acquired and other information on environmental and environmental risks.
Article 16 Where an application is made for a simple registration, the applicant shall submit the following materials:
(1) The application form for simple registration;
(ii) The physicochemical properties of new chemicalsubstances, as well as ecotoxicological test reports or data such as persistence, bioaccumulation and aquatic environmental toxicity;
(3) The commitment letter of commitment to implement or transmit environmental risk control measures shall be signed by the legal representative of an enterprise or institution or its authorized person and sealed with a public seal.
The ecological toxicology test report stipulated in ARTICLE 14 shall include the test data completed by the test organisms of the People's Republic of China in accordance with the relevant standards.
In addition to the application materials specified in the preceding paragraph, the applicant shall also submit other information on the environmental and health hazards of the new chemicalsubstances he has acquired and other information on environmental hazards and environmental risks.
Article 17 The same applicant may apply for registration for environmental management of new chemical substances with similar molecular structures, similar uses or similar test data. The number of applications for registration is determined by the sum of the registrations for each substance.
If two or more applicants apply for the same new chemical environmental management registration at the same time, they may jointly submit the application materials for joint registration of the new chemical environmental management. The number of applications for registration is determined on the basis of the sum of the number of applications for registration per applicant.
Article 18 After receiving the application materials for environmental management registration of new chemical substances, the competent department of ecology and environment under the State Council shall deal with them according to the following circumstances:
(1) If the application materials are complete and conform to the legal form, or the applicant submits all the correction application materials as required, it will be accepted;
(2) If there are errors in the application materials that can be corrected on the spot, the applicant is allowed to make corrections on the spot;
(3) If the applied substance does not need to carry out environmental management registration of new chemical substances, or there are other circumstances in the application materials that are not accepted by laws and regulations, it shall make a decision of non-admission on the spot or within five working days;
(4) If there are situations where the applicant and its agent do not comply with the provisions of these Measures, the application materials are incomplete, and other legal forms are not met, the applicant shall be notified on the spot or within five working days of the entire content that needs to be corrected. If the application is not notified within the time limit, it will be accepted as of the date of receipt of the application materials
Article 19 After accepting the application for routine registration, the competent department of ecological environment under the State Council shall organize an expert committee and the technical institution for the environmental management of chemical substances to conduct a technical evaluation. The technical review should focus on the following:
(1) the name and identification of the new chemical substances;
(2) the quality of the test report or information on new chemical substances;
(3) the environmental and health hazard characteristics of new chemical substances;
(4) Environmental exposure and environmental risks of new chemical substances;
(5) Whether to implement new-use environmental management when included in the List of Existing Chemical Substances in China;
(6) whether environmental risk control measures are appropriate;
(7) The necessity of applying for high-hazard chemical substances;
(8) The necessity of the protection of trade secrets.
The technical review should include the conclusions of the review of the content of the preceding paragraph, as well as the recommendations on whether registration should be granted and recommendations on environmental management requirements.
If the application materials submitted by the applicant are found to be not in accordance with the requirements or are not sufficient to make a comprehensive assessment of the environmental risks of new chemical substances, the competent department of ecological environment under the State Council may request the applicant to provide additional test reports or information.
Article 20 After accepting the application for simple registration, the competent department of ecological environment under the State Council shall organize the technical institution for the environmental management of chemical substances to which it belongs to conduct a technical evaluation. The technical review should focus on the following:
(1) the name and identification of the new chemical substances;
(2) the quality of the test report or information on new chemical substances;
(3) the persistence, bioaccumulation and toxicity of new chemical substances;
(4) The cumulative environmental risks of new chemical substances;
(5) The necessity of the protection of trade secrets.
The technical review opinion sits on the evaluation of the content seamount in the preceding paragraph and the recommendation on whether registration should be granted.
If the application materials submitted by the applicant are found to be not in accordance with the requirements, the competent department of ecological environment under the State Council may request the applicant to provide additional relevant test reports or information.
Article 21 The competent department of ecological environment under the State Council shall examine the technical evaluation opinions of the regular registration and make a decision on the following circumstances:
(1) If unreasonable environmental risks are not found, they shall be registered and a conventional registration certificate for environmental management of new chemical substances shall be issued to the applicant (hereinafter referred to as the regular registration certificate). The issuance of a conventional registration certificate for high-hazard chemical substances shall also meet the requirements of the necessity of the application activities;
(2) If there is an unreasonable environmental risk found, or does not meet the requirements for the necessity of applying for a high-hazard chemical substance, it shall not be registered, and the applicant shall be notified in writing and the reasons shall be explained.
Article 22 The competent department of ecological environment under the State Council shall examine the technical evaluation opinions on simple registration and make a decision on the following circumstances:
(1) If no one has been found to be persistent, bioaccumulative and toxic, and no cumulative environmental risk sourcing is found, and a simple registration certificate for environmental management of new chemical substances is issued to the applicant (hereinafter referred to as a simple registration certificate);
(2) If it does not meet the registration conditions stipulated in the preceding paragraph, it shall not be registered, and the applicant shall be notified in writing and the reasons given.
Article 23 If any of the following circumstances occur, the competent department of ecological environment under the State Council shall not register, notify the applicant in writing and explain the reasons:
(1) using deception such as concealment or providing false materials in the course of registration application;
(2) failing to provide the relevant test reports or information within six months if the relevant test reports or information are not provided in accordance with the requirements of Article 19 (3) or Article 20, paragraph 3, of these Measures;
(3) Other circumstances in which the laws and regulations provide for non-registration.
Article 24 Before making a registration decision, the competent department of ecological environment under the State Council shall make public the names or types of new chemical substances to be registered, the applicant and his agent, the type of activity and the requirements for environmental management for new uses. The period of publicity shall not be less than three working days.
Article 25 After accepting the application for registration of new chemical sisanother environmental management, the competent department of ecological environment under the State Council shall initiate the technical evaluation work in a timely manner. The technical review time of the regular registration shall not exceed 60 days, and the technical evaluation time of the simple registration shall not exceed 30 days. Where the competent department of ecological environment under the State Council notifies the supplementary provision of relevant test reports or materials, the time required for the applicant to supplement the relevant materials shall not be included in the time limit for technical evaluation.
The competent department of ecological environment under the State Council shall, within twenty working days from the date of acceptance of the application, make a decision on whether to register it. If a decision cannot be made within twenty working days, it may be extended by ten working days with the approval of the person in charge of the ecological environment department under the State Council, and the applicant shall be informed of the reasons for the extension.
The time for technical evaluation shall not be included in the time limit for approval as specified in the second paragraph of this article.
Article 26 The registration certificate shall specify the following items:
(1) Type of registration certificate;
(2) The name of the applicant and its agent;
(3) Identification information such as Chinese and English names or class names of new chemical substances;
(4) Application purpose;
(5) Number of applications for registration;
(6) Types of activities;
(VII) Environmental risk control measures.
For high-hazard chemical substances and new chemical substances with persistence and bioaccumulation, or with persistence and toxicity, or with bioaccumulation and toxicity, the regular registration certificate should also specify one or more of the following environmental management requirements:
(1) Limit the discharge amount or concentration of new chemical substances;
(2) Requirements for implementing new-use environmental management when listed in the "Inventory of Existing Chemical Substances in China";
(3) Submit annual report;
(4) Other environmental management requirements.
Article 27 After the application for registration of environmental management of new chemical substances is accepted, the applicant may withdraw the registration application according to law before the decision of the competent department of ecology and environment under the State Council.
Article 28 After the competent department of ecology and environment under the State Council makes a decision on the environmental management registration of new chemical substances, it shall disclose the environmental management registration of new chemical substances within 20 working days, including the name or class name of the registered new chemical substance, and the applicant Information about its agents, types of activities, and environmental management requirements for new uses.
Article 29 Before a new chemical substance that has obtained a regular registration certificate is listed in the "Inventory of Existing Chemical Substances in China" in accordance with Article 44 of these Measures, the holder of the registration certificate shall have one of the following circumstances Should apply for registration again:
(1) The quantity of production or import intends to exceed the amount applied for registration;
(2) The type of activity to be converted from import to production;
(3) To change the application purpose of the chemical substance;
(4) Plan to change environmental risk control measures;
(5) Other circumstances that lead to increased environmental risks.
When re-applying for registration, the applicant shall submit re-registration application materials, explain the reasons for the change of relevant matters, re-compile and submit an environmental risk assessment report, and focus on explaining the environmental risk control measures to be taken after the change and their appropriateness, and whether they exist Unreasonable environmental risks.
Article 30 Before new chemical substances that have obtained regular registration certificates are included in the "Inventory of Existing Chemical Substances in China" in accordance with the provisions of Article 44 of these Measures, except for the circumstances specified in Article 29 of these Measures, If any other information stated in the registration certificate changes, the holder of the registration certificate shall apply for the change of the registration certificate.
For new chemical substances that have obtained a simple registration certificate, if the information stated in the registration certificate changes, the holder of the registration certificate shall apply for the change of the registration certificate.
Applying for the change of the registration certificate, the applicant shall submit the reason for the change and relevant certification materials. Among them, if it is proposed to update the Chinese and English names of chemical substances or the CAS number (CAS) and other identification information, the certification materials should fully demonstrate that the chemical substances before and after the change belong to the same chemical substance.
The State Council department in charge of ecology and environment accepts and organizes technical reviews with reference to the simple registration procedure and time limit, and makes a decision to change the registration certificate. Among them, for the proposed updating of Chinese and English names of chemical substances or chemical abstract service number (CAS) and other identification information, the competent department of ecology and environment of the State Council may organize an expert committee to conduct a technical review; for those who cannot determine whether the chemical substances belong to the same chemical substance before and after the change , The change will not be approved.
Article 31 The following chemical substances listed in the "Inventory of Existing Chemical Substances in China" in accordance with Article 44 of these Measures shall be subject to new-use environmental management:
(1) Highly hazardous chemical substances;
(2) Chemical substances with persistence and bioaccumulation, or with persistence and toxicity, or with bioaccumulation and toxicity.
If the holder of the registration certificate changes the use of a high-hazard chemical substance, or if someone other than the holder of the registration certificate uses it for industrial purposes, it shall report to the competent department of ecology and environment under the State Council before production, import or processing. Apply for new environmental management registration.
For the chemical substances listed in Paragraph 2 of Paragraph 1 of this Article, if they are intended to be used for industrial purposes other than the permitted uses stipulated in Article 44 of these Measures, they shall apply to the competent department of ecology and environment of the State Council before production, import or processing. Go through the registration of environmental management for new purposes.
Article 31 The following chemical substances listed in the "Inventory of Existing Chemical Substances in China" in accordance with Article 44 of these Measures shall be subject to new-use environmental management:
(1) Highly hazardous chemical substances;
(2) Chemical substances with persistence and bioaccumulation, or with persistence and toxicity, or with bioaccumulation and toxicity.
If the holder of the registration certificate changes the use of a high-hazard chemical substance, or if someone other than the holder of the registration certificate uses it for industrial purposes, it shall report to the competent department of ecology and environment under the State Council before production, import or processing. Apply for new environmental management registration.
For the chemical substances listed in Paragraph 2 of Paragraph 1 of this Article, if they are intended to be used for industrial purposes other than the permitted uses stipulated in Article 44 of these Measures, they shall apply to the competent department of ecology and environment of the State Council before production, import or processing. Go through the registration of environmental management for new purposes.
Article 32 For an application for new-use environmental management registration, the applicant shall submit a new-use environmental management registration application form and the environmental exposure assessment report and environmental risk control measures of the chemical substance for new use and other materials. For high-hazard chemical substances, socio-economic benefit analysis materials should also be submitted to fully demonstrate the necessity of the substance for the application for registration.
After receiving the application materials, the department in charge of ecology and environment under the State Council accepts and organizes the technical review in accordance with the normal registration procedures, processes them according to the following circumstances, and notifies the applicant in writing:
(1) If no unreasonable environmental risks are found, they shall be registered. For high-hazard chemical substances, it should also meet the requirements for the necessity of the application;
(2) If there is an unreasonable environmental risk or does not meet the requirements for the use of high-hazard chemical substances, the application shall not be registered.
After making an environmental management registration decision for a new purpose, the competent department of ecology and environment under the State Council shall, within 20 working days, disclose the name of the applicant and its agent, the name or class name of the chemical substance involved, the new use registered, and the corresponding Environmental risk control measures and environmental management requirements. Among them, those that do not belong to high-hazardous chemical substances will be added to the "Chinese Inventory of Existing Chemical Substances" for which new permitted uses have been registered; those that belong to high-hazardous chemical substances will be listed in the "Chinese Existing Chemical Substances List 》The scope of the new-use environmental management remains unchanged.
Article 33 After obtaining the registration certificate, the applicant may apply to the State Council department in charge of ecology and environment to cancel the registration certificate.
Article 34 In one of the following situations, for the sake of public interest, the State Council department in charge of ecology and environment may change or withdraw the registration certificate in accordance with the relevant provisions of the Administrative License Law of the People’s Republic of China:
(1) It needs to be changed or withdrawn in accordance with Article 42 of these Measures;
(2) The content of environmental management registration of new chemical substances does not comply with the national industrial policy;
(3) Changes in relevant laws, administrative regulations or mandatory standards;
(4) The content of environmental management registration of new chemical substances conflicts with the requirements of international treaties concluded or participated in by the People's Republic of China;
(5) Other circumstances that should be changed or withdrawn as required by laws and regulations.
Article 35 In one of the following circumstances, the competent department of ecology and environment under the State Council may revoke the registration certificate in accordance with the relevant provisions of the Administrative License Law of the People’s Republic of China:
(1) The applicant or its agent obtains the registration certificate by fraud, bribery or other improper means;
(2) A staff member of the State Council department in charge of ecology and environment abuses his power, neglects his duties, or violates the legal procedures to issue a registration certificate;
(3) Other circumstances that should be revoked as required by laws and regulations.
Article 36 For handling the environmental management filing of new chemical substances, the filing form and supporting materials that meet the corresponding circumstances specified in Article 10, paragraph 3 of these Measures shall be submitted, and the environmental and health status of the new chemical substances already in its possession shall be submitted together.
Article 37 After receiving the filing materials for the environmental management of new chemical substances, the competent department of ecological environment under the State Council shall file the complete and complete filing materials and send a receipt for the record. After the applicant submits the filing materials, he or she may carry out the activities related to the new chemical substances in accordance with the contents of the filing.
When the filing of new chemical sisphons or related information changes, the applicant shall promptly change the filing information.
The competent department of ecological environment under the State Council shall regularly publish the record of environmental management of new chemical substances.
Article 38 Producers, importers and processing users of new chemical substances shall convey the following information to downstream users:
(1) the registration certificate number or the record receipt number;
(2) The application for use of new chemical substances;
(3) the environmental and health hazard characteristics of new chemical substances and environmental risk control measures;
(4) Environmental management requirements for new chemical substances.
Users of new chemicals may request the supplier to provide information about the new chemicalsubstances specified in the preceding paragraph.
Article 39 Researchers, producers, importers and processing users of new chemical substances shall establish a system for recording the activities of new chemical substances, truthfully record the time, quantity and use of the activities of new chemical substances, and implement environmental risk control measures and environmental management requirements.
Relevant information such as routine and simple registration materials and records of the activities of new chemical substances shall be kept for at least 10 years. Relevant materials such as filing materials and records of the activities of new chemical substances shall be kept for at least three years.
Article 40 Producers and processing users of the regular registration of new chemical substances shall implement environmental risk control measures and environmental management requirements and disclose the implementation of environmental risk control measures and environmental management requirements through their official websites or other means that are easily known to the public.
Article 41 The holder of the registration certificate shall report the first activity of the new chemical substance to the competent department of ecological environment under the State Council within 60 days from the date of the first production or within 60 days from the date of the first import and transfer to the processed user.
If the environmental management requirements as set out in the regular registration certificate stipulate that the requirements for the submission of annual reports, the holder of the registration certificate shall, from the following year of registration, report to the competent department of ecological environment under the State Council on the actual production or import of new chemical substances approved for the previous year, the discharge to the environment, and the implementation of environmental risk control measures and environmental management requirements by April 30 of each year.
Article 42 If researchers, producers, importers and processing users of new chemical substances discover that new chemical substances have new environmental or health hazard characteristics or environmental risks, they shall promptly report them to the competent department of ecological environment under the State Council; if they may lead to an increase in environmental risks, they shall take timely measures to eliminate or reduce environmental risks.
The competent department of ecological environment under the State Council may request relevant researchers, producers, importers and processing users to further submit relevant environmental or health hazard and environmental exposure data information on new chemical substances that may continue to increase in environmental risks, in accordance with the national registration of new chemical sisphons, actual production or import, discharge to the environment, and newly discovered environmental or health hazard characteristics.
After receiving the relevant information, the competent department of ecological environment under the State Council shall organize the technical institution situated in the environmental management of chemical substances and the expert committee to conduct technical evaluation;
Article 43 The competent department of ecological environment under the State Council shall inform the competent department of ecological environment at the provincial level of the registration of environmental management of new chemical substances, environmental risk control measures and environmental management requirements, first activities and annual reports; the competent department of ecological environment at the provincial level shall inform the municipal ecological environment department in the district.
The competent department of ecological environment at or above the municipal level of the district shall conduct a supervision and spot check on whether the producers, importers and processing users of new chemical substances shall, as required, handle the registration of the environmental management of new chemical substances, the authenticity of the registration matters, the matters specified in the registration certificate and the implementation of other relevant provisions of these Measures.
Researchers, producers, importers and processing users of new chemical substances shall truthfully provide relevant information and be subject to supervision and spot checks by the competent ecological environment department.
Article 44 If a new chemical substance that has obtained a regular registration certificate has reached five years from the date of its first registration, the State Council department in charge of ecology and environment shall include it in the "Inventory of Existing Chemical Substances in China" and make an announcement.
For new chemical substances that are persistent and bioaccumulative, or persistent and toxic, or bioaccumulative and toxic, when they are listed in the "Inventory of Existing Chemical Substances in China", their permitted uses should be indicated.
For high-hazard chemical substances and new chemical substances with persistence and bioaccumulation, or persistence and toxicity, or bioaccumulation and toxicity, when listed in the "Inventory of Existing Chemical Substances in China", it shall be stipulated that in addition to the annual report Environmental management requirements.
The provisions of the first three paragraphs of this article apply to the routine registration of new chemical substances that apply for cancellation in accordance with Article 33 of these Measures.
New chemical substances for simple registration and filing, as well as routinely registered new chemical substances that are withdrawn or withdrawn in accordance with Articles 34 and 35 of these Measures, are not included in the "Inventory of Existing Chemical Substances in China".
Article 45. New chemical substances that have obtained regular declaration registration certificates in accordance with the "Administrative Measures on Environmental Protection of New Chemical Substances" (Ministry of Environmental Protection Order No. 7), if they have not been listed in the "Inventory of Existing Chemical Substances in China", they shall be included in the "Inventory of Existing Chemical Substances in China" Five years from the date of the first production or import activity or five years from the date of implementation of these Measures.
According to the "Administrative Measures on New Chemical Substances" (Decree No. 17 of the State Environmental Protection Administration), new chemical substances that have been registered for environmental management and have not been listed in the "Inventory of Existing Chemical Substances in China" shall be subject to these Measures Within six months from the date of implementation, be included in the "Inventory of Existing Chemical Substances in China".
If these measures have been included in the "Inventory of Existing Chemical Substances in China" and the protection of identification information such as substance names is implemented before the effective date of this method, the protection period of the identification information shall be up to December 31, 2025.
Article 46 Anyone who, in violation of the provisions of these Measures, obtains the registration of environmental management of new chemical substances by improper means such as deception or bribery shall be ordered by the competent department of ecological environment under the State Council to make corrections, impose a fine of 10,000 yuan to 30,000 yuan or less, and carry out joint punishment for breach of trust in accordance with the law, and shall no longer accept its application for the registration of new chemical environmental management within three years.
Article 47 If any of the following acts are violated by these Measures, the competent department of ecological environment under the State Council shall order the correction and impose a fine of not more than 10,000 yuan; if the circumstances are serious, joint disciplinary action for breach of trust shall be carried out in accordance with the law, and the application for registration of environmental management of new chemical substances shall not be accepted within one year:
(1) failing to report the first activity of new chemical substances as required or being allowed to register the actual production or import of new chemical substances in the previous year, as well as the implementation of environmental risk control measures and environmental management requirements;
(2) failing to report new environmental or health hazard characteristics or environmental risk information of new chemical substances as required, or failing to take measures to eliminate or reduce environmental risks, or failing to submit information on environmental or health hazards or environmental exposure data.
Article 48 If any of the following acts are committed in violation of the provisions of these Measures, the competent department of local ecological environment at or above the municipal level of the district shall order the correction and impose a fine of not less than 10,000 yuan and 30,000 yuan; if the circumstances are serious, joint disciplinary action for breach of trust shall be carried out in accordance with the law, and the application for registration of environmental management of new chemical substances shall not be accepted within one year:
(1) producing or importing new chemical substances without a registration certificate, or processing and using new chemical substances without a registration certificate;
(2) failing to re-register the production or import of new chemical substances in accordance with the provisions;
(3) The chemical substances that have not been approved after the registration of environmental management for new uses by the competent department of ecological environment under the State Council shall be used for industrial purposes other than those permitted.
Article 49 If any of the following acts are committed in violation of the provisions of these Measures, the competent department of local ecological environment at or above the municipal level of the district shall order it to make corrections within a time limit and impose a fine of not less than 10,000 yuan and not more than 30,000 yuan; if the circumstances are serious, joint disciplinary measures for breach of trust shall be carried out in accordance with the law, and the application for registration of environmental management of new chemical substances shall not be accepted within one year:
(1) failing to file a record, or producing or importing new chemical substances in accordance with the information for the record, or processing and using new chemical substances that have not been filed;
(2) failing to produce, import or process the use of new chemical substances in accordance with the provisions of the registration certificate;
(3) failing to register changes, or producing or importing new chemical substances in accordance with the changes;
(4) failing to implement the relevant environmental risk control measures or environmental management requirements, or failing to disclose the relevant information in accordance with the provisions;
(5) failing to transmit the required information to the downstream users, or refusing to provide relevant information on the new chemical substances;
(6) failing to establish a system for recording the activities of new chemical substances, or failing to record the activities of new chemical substances or keeping relevant information;
(7) Failure to implement the environmental management requirements set out in the List of Existing Chemical Substances in China.
Article 50 If a member of the Expert Committee cheats in the registration and evaluation of the new chemical environmental management, or has other acts of dereliction of duty, resulting in serious infact of the evaluation results, the competent department of ecological environment under the State Council shall cancel its membership of the expert committee and make it public to the public.
Article 51 If a testing institution that provides test data for a new chemical substance application issues a false report, the competent department of ecological environment under the State Council shall impose a fine of 10,000 yuan or less on the testing institution, a fine of 10,000 yuan to 30,000 yuan or less for the person in charge and other persons directly responsible for the testing institution, and shall carry out joint disciplinary punishment for breach of trust in accordance with the law, and shall not accept the test report issued by the testing institution or the relevant responsible person to participate in the test report within three years.
Chapter VI Supplementary Provisions
Article 52 The meaning of the following terms in these measures:
(1) Environmental risk refers to the degree and probability of harmful effects on the environment and health after chemical substances with environmental or health hazard properties enter or may enter the environment during production, processing, use, disposal. Risks caused by emergencies such as production safety accidents and transportation accidents.
(2) High-hazard chemical substances refer to chemical substances that are both persistent, bioaccumulative, and toxic, as well as chemical substances that are both highly persistent and bioaccumulative, or other chemical substances with equivalent environmental or health hazards. .
(3) Processing and use of new chemical substances refers to the use of new chemical substances for production and operation activities such as packaging, preparation, or manufacturing. It does not include trading, warehousing, transportation and other business activities and the use of articles containing new chemical substances.
Article 53 The environment for new chemical substances has been handled in accordance with the provisions of the “Administrative Measures for New Chemical Substances” (Order No. 7 of the Ministry of Environmental Protection) and the “Administrative Measures for New Chemical Substances (Order No. 17 of the State Environmental Protection Administration) For management registration, the relevant registration will continue to be effective after the implementation of these measures.
Article 54 The Measures shall be interpreted by the competent department of ecology and environment under the State Council.
Article 55 These Measures shall come into force on January 1, 2021, and the "Administrative Measures on New Chemical Substances" issued by the former Ministry of Environmental Protection (Ministry of Environmental Protection Order No. 7) shall be repealed at the same time.
|
|
MEE Order No. 12 |
EU-REACH |
|
Substances to be registered |
Substances not listed in IECSC, regardless its manufactured / imported quantities in China |
Substances manufactured / imported in the EU/EAA |
|
Polymer |
Registration requirement for polymers as well, as long as it is defined as new substance |
The polymer itself is exempted from registration |
|
Only Representative (OR) |
Non-Chinese company (incl. distributor) who exports new substances to China can appoint OR |
Only non-EU/EEA manufacturer, formulator, or article producer can appoint OR |
|
Registration Type/Form |
Record Filing, Simplified Registration, Regular Registration / Individual Registration, Series Registration, and Joint Registration |
Standard (Full) Registration and reduced Registration (intermediate) /Joint Submission and Individual Submission |
|
Data Sharing |
No Substance Information Exchange Forum (SIEF) and/or data sharing are not mandatory |
SIEF. Testing on vertebrate animals as last resort. It is necessary to take measures limiting duplication of other tests. |
|
Data Requirements for Registration |
Physicochemical properties, toxicity, and ecotoxicity data requirements depend on 2 tonnage levels (1-10 TPA and > 10 TPA). No test requirement for Record Filing (Q<1 TPA, polymer with NM<2% PLC) Additional data requirement for highly hazardous substances (e.g. PBT/vPvB) and PB/PT/BT substances. Some ecotoxicological tests must be performed by using local test organism (e.g. Gobiocypris rarus, activated sludge) in China
|
Physiochemical properties, toxicity and ecotoxicity data requirement depend on 4 tonnage levels (1-10 TPA, 10-100 TPA, 100-1000 TPA, > 1000 TPA) |
|
GLP Qualification |
Because of not being OECD member, China GLP test data is not accepted as international GLP data under EU-REACH and other non-Chinese chemical regulations. |
Toxicity and ecotoxicity tests shall be performed according to internationally accepted GLP Norm. |
|
Language |
Predominantly Chinese. Test reports in English are possible, but Chinese translation for the summary must be provided. |
English |
|
Software |
No specific software. Submission by using SCC’s online registration system. |
IUCLID submission to ECHA through REACH-IT |
|
Registration Fee to Authority |
No registration fee required by SCC. Enquiry for full IESCS check by SCC currently 3,000 CNY per substance. |
Commission Implementing Regulation (EU) 2015/864 |
China's State Council published the final version of the COsmetic Supervision and Administration Regulation (CSAR) and this came into force on 1 January 2021 and replaced the Cosmetic Hygiene Supervision REgulations. CSAR regulates all cosmetics and cosmetic raw materials in China. All the manufacturers, importers and exporters must ensure that their cosmetic products and raw ingredients meet the compliance obligations under CSAR before producing, importing, and exporting business.
Substances are divided into two categories:
- cosmetic raw ingredients
- cosmetic products.
Based on different types of substances, different submission procedures (record filing and registration) apply.
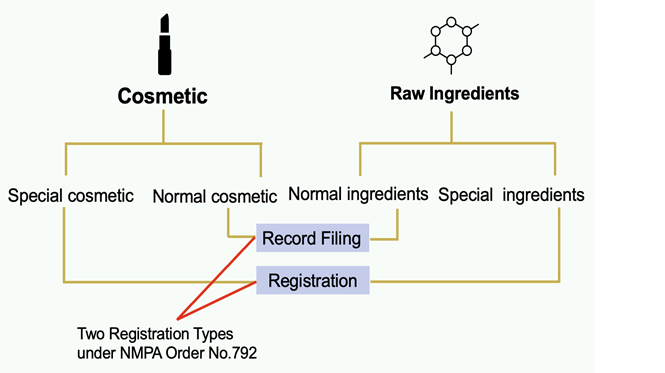
Cosmetic raw material is defined as natural or synthesis ingredients which are used in making cosmetic products. According to the requirements, existing ingredients need to do record filing submission, while new ingredients must do a formal registration to produce or place in China. The distinguished point for checking the procedure for ingredients Is to check if it falls within the cosmetic inventory.
IECIC stands for Inventory of Existing Cosmetic Ingredients in China. Currently, it contains 8972 existing cosmetic ingredients. In addition to the main inventory, there are seven cosmetic inventory lists that can be used in parallel with IECIC. If ingredients are found on the inventory, then it only needs to submit record filing.
|
Inventory lists |
Number listed |
Obligation under list |
|
List of banned ingredients in cosmetics (2021) |
1284 |
Not allow to use |
|
List of banned plant (animal) ingredients in cosmetics (2021) |
109 |
Not allow to use |
|
List of restricted substances in cosmetics (2021) |
47 |
Meet certain requirements |
|
List of preservatives allowed in cosmetics (2015) |
51 |
Allow to use |
|
List of sunscreen agents allowed in cosmetics |
27 |
Allow to use |
|
List of colorants allowed in cosmetics (2015) |
157 |
Allow to use |
|
List of hair dyes allowed in cosmetics (2015) |
75 |
Allow to use |
|
IECIC (2021) |
8972 |
Register / Record Filing |
New raw ingredients are defined as cosmetic ingredients that are not listed in the IECIC inventory.
Applicants need to submit the following documents to comply with CSAR:
- Applicant’s details which include name, address, and contact.
- Research and Development report which addresses the sources and properties of the ingredients, basis of efficacy, data, etc.
- Quality control standard. It needs to explain the process during the production of the cosmetic ingredient, quality specification index, testing methods, and possible risks.
- Safety evaluation report. Toxicological safety evaluation data, evaluation, and necessary toxicological test data must be given in this report.
The application may take up to 4 months before a decision is made. The technical committee will need around 3 months to undertake the evaluation work.
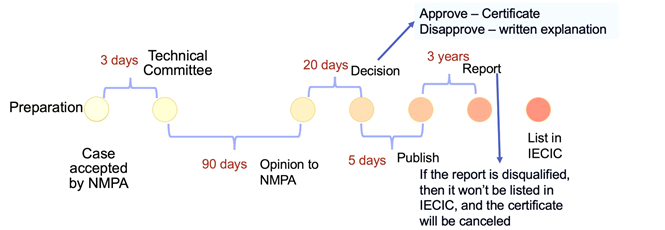
The registration timeline of cosmetic products is the same as cosmetic ingredients registration. But the submission documents are different. For cosmetic products registration, applicants need to prepare:
- The name, address, and contact information of the registration applicant filing person and manufacturer.
- product name, formula, or full product ingredients.
- Standards implemented by the product.
- Product label sample.
- Product inspection report.
- Product safety assessment data.
- Registrant qualification
For imported cosmetics, they must be labeled in Chinese and contain the following information:
- Product name, special cosmetics registration certificate number
- The name and address of the registrant, recorder, and entrusted production enterprise
- Cosmetic production license number
- The standard number of the product
- All ingredients
- Net weight
- Use period, method of use and necessary safety warnings
CSAR also introduces the annual reporting requirements. Applicants should submit the annual report of cosmetics from January 1 to March 31 each year.
- Basic information and production of new cosmetic ingredients.
- Information on cosmetic registrants, filers, or entrusted manufacturers who use new raw materials to produce cosmetics.
- Information on cosmetics using new raw materials, including product name, product registration or filing number, number of products produced or imported, sold, etc.
- Sampling inspection, investigation, and recall of cosmetics produced with new raw materials.
- The adverse reaction monitoring system, statistical analysis of adverse reactions, and measures of cosmetics produced with new cosmetic ingredients for cosmetics manufacturers.
- Risk monitoring and evaluation management system and measures of cosmetics produced with new cosmetic ingredients for cosmetics manufacturers.
On December 31, 2021, China’s National Medical Product Administration (NMPA) launched an online cosmetic ingredient submission platform. This platform, Cosmetic Ingredients Safety Information Registration Platform, will require companies handing existing cosmetic ingredients to submit information. The platform is created based the requirements of CASR (state council order No.727). For foreign cosmetic ingredient manufacturers, they can create their own accounts to submit information, or authorize representative companies to submit ingredient information on their behalf.
Cosmetics Raw Material manufacturers, domestic or overseas, are required to submit to the Cosmetic Ingredients Safety Information Registration Platform. Manufacturers can submit information by themselves, or they can appoint an authorized representative to submit and maintain ingredient safety information on their behalf. This applied to cosmetic raw materials regardless of its tonnage.
This applies to two types of substances:
-
Substance that are included in IECIC (Chemical Inventory, Inventory of Existing Chemical Substances Produced or Imported in China) but not listed in IECSC (Inventory of Existing Cosmetic Ingredients Produced or Imported in China)
-
Substances that are listed in IECIC and IECSC.
Basic information and manufacturing process
-
Trade name
-
Composition
-
Basic properties
-
Purpose of use
-
Suggested percentage in cosmetics
-
Restrictions on the use of raw materials
-
Properties
-
Physical and chemical properties
-
Brief statement of the Manufacturing process
Quality control requirements and characteristic indicators
-
Identification method
-
Quality and characteristics of ingredients
Limit requirements for risky substances
- Heavy metal
-
Microbiology indicators
-
Pesticide residue risk
-
Others
Assessment conclusions of international authorities
Brief description of the requirements for use in other industries
Other issues to be explained
The China Food & Drug Administration (CFDA), now called the National Medical Products Administration (NMPA), is responsible for medical devices, drugs, and healthcare services. The organization is headquartered in Beijing, with offices in each province. The Centre for Medical Device Evaluation (CMDE) is responsible for conducting the dossier review during the medical device registration process. The General Administration of Quality Supervision, Inspection, and Quarantine (AQSIQ) conducts mandatory safety registration, certification, and inspection for certain devices.
China has National Standard for medical devices. Any medical device must follow the Chinese National Standard Specifications. More than 35% of all the IEC and ISO standards have now been adopted by China, but many are not direct transpositions and contain China specific requirements.
Primary medical device related international standards and their Chinese equivalents.
-
The first step in registering your medical device in China is to classify your product in China. China classifications range from Classes 1-3.
*Note: If the medical product is registered as a Class 2 product in the US or EU, it does not mean it will be a Class 2 product in China.
-
The second step in registering the medical device in China is local type testing. This requires the device manufacturer to send a sample(s) of the product to China, where one of the NMPA testing centres in China will perform local type testing.
-
The third step for Class 2 and Class 3 products is to determine whether a clinical evaluation report (CER) or a local clinical study will be needed for China device approval. Hopefully a CER will suffice, but if not, a local clinical trial may be needed. Local clinical trials in China can be expensive and normally take 1-1.5 years.
-
In June 2021, the NMPA issued a new set of regulations that will make it easier for innovative medical devices for the treatment of urgent public health emergencies and rare diseases to obtain approval in China.
-
Certain products will be exempt from clinical trials in China if their manufacturers can demonstrate their safety and efficacy in other markets. All medical devices require CFDA registration prior to being sold in China.
-
The Chinese State Council released the new Regulations for the Supervision and Administration of Medical Devices in 2014. Compared with the old regulations (48 articles), the new ones have 80 articles and many changes on device registration; clinical trials; adverse events; recalls, etc.
-
The new regulations are consistent with the goal of the “National 12th five-Year Plan” to foster innovation and encourage domestic companies’ research and development while enhancing the protection of public health.
- Product Risk Analysis Document
- Product Technical Specification
- Product Testing Report (company’s self-testing report or 3rd party report)
- Clinical Evaluation Report
- Key Manufacturing Information (process, flowchart, material, etc.)
- Design/artwork of IFU and product label for the minimum selling unit
- Legal Documents
- Legal qualification of the foreign manufacturer (i.e. ISO 13485)
- Market authorization approval at the country of origin (i.e. CFG+510k or CE)
- Authorization letter to the agent in China.
|
Device Class |
Timeline |
Cost |
Period of Validity |
|
Class I |
Immediately |
Free: Initial/change/extension |
NA |
|
Class II |
|
RMB 210,900 / 42,000 / 40,800 (approx. EUR 26,500 / 5,300 / 5,150)
|
5 years |
|
Class III |
|
RMB 308,800 / 50,400 / 40,800 EUR 38,900 / 6,350 / 5,150
|
5 years |
-
Identification of compliance requirements under various guidelines including all data requirements.
-
Data gap analysis and pre-assessment support
-
Technical documentation support
-
Pre and post submission support and technical liaison with authorities.
- National Level
- The Ministry of Health (MOH); is responsible for food hygiene, chemical contamination, food borne disease control, permission and inspection for new food, new food contact articles and food contacts additives notification approval, preparation/revision of food standards.
- State Administration of Food and Drug (SFDA) is responsible for monitoring and supervision of the actual implementation of food safety regulation which also includes coordination.
- Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China (AQSIQ) is responsible for granting the permission for production of food and food packaging products; and imported food contact material compliance verification.
- Province Level
- MOH Local Departments: responsible for supervision and inspection. No FDA involvement
Also in Shanghai, The FDA has the main responsibility to ensure that China MOH food contact material requirements and AQSIQ’s food contact material requirements are implemented
- The level of substance migrated from the FCM into foods should not be harmful to human health under the recommended use conditions.
- When FCM comes in contact with food particles under the recommended conditions, they should not cause changes in food composition, structure or attributes such as colour, odour or taste; they should not have any technical effect on the food particles.
- On the assumption that the desired objectives may be accomplished, the quantity of chemicals utilised in food contact materials and articles should be limited.
- Non-intentionally added chemicals in goods must be monitored by producers of food-contact materials and articles to ensure that the migrating quantity satisfies the stipulated requirements.
- For substances that do not contact foods and have an effective resistant/proofing layer between such substance and the foods, or for substances that are not listed in the appropriate national food safety standards, producers of food-contact materials and articles should conduct safety assessments and control measures to ensure that the migrating quantity of such substances does not exceed 0.01mg/kg. Nanomaterials, as well as carcinogenic, teratogenic, and mutagenic substances, are exempt from the aforementioned principles, but must follow all applicable laws, rules, and norms.
- Producers must establish Traceability systems for FCM’s from production to distribution.
When food contact products are sold, they must be accompanied by sufficient product information. This comprises the product name, material, declaration of conformance to applicable laws, regulations, and standards; the producer's and/or dealer's names, addresses, and contact information; the production date, warranty duration (if applicable), instruction for use, and qualification certificate.
Apart from the points discussed above there are some specific points/things that need to be present while the labelling of the article
- Name of the Food
- Specific List of Ingredients
- Stated as the descending order of the composition of that particle chemical/substance.
- List of Compound Ingredients (i.e. when two or more ingredients make up an ingredient) must be declared if any and if >5%.
- Added water; except in cases where water is in fact a part of the ingredient and does not evaporate during the manufacturing process.
- If the package contains a mixture of substances; clear labelling must be done for all the basic as well as compound ingredients if any.
- Nutritional Information (i.e. nutritional facts per serving/ per 100mg)
- It must include energy value(kcal).
- Fat Declaration
- Presence of hydrogenated vegetable fats or bakery shortening should be declared as they contain trans fat
- Food Additive Declaration
- The following class titles shall be used in conjunction with the specific names or accepted international numerical identifications for food additives falling into the appropriate classes and appearing in lists of food additives approved for use in foods generally:
- Acidity Regulator
- Acids
- Anticaking Agent
- Antifoaming Agent
- Antioxidant
- Unnecessary extra colouring materials that must be noted on the label – When an extraneous colouring matter has been introduced to any food product, one of the following phrases in capital letters must be shown.
- The following class titles shall be used in conjunction with the specific names or accepted international numerical identifications for food additives falling into the appropriate classes and appearing in lists of food additives approved for use in foods generally:
CONTAINS PERMITTED NATURAL COLOUR(S) OR CONTAINS PERMITTED SYNTHETIC FOOD COLOUR(S). Similarly, for added flavours the label must contain:
- CONTAINS ADDED FLAVOUR
-
- In case the article contains both colour and added flavours then both the points stated above must be declared on it.
- Name and Complete Address of the manufacturer
- Net Quantity
- Lot/Code/Batch Identification
- Date of Manufacture or packing
- Best Before and Use by Date
- Instructions for Use
- The use of raw materials in food-contact materials and articles shall be in accordance with the provisions of applicable national food safety regulations and public announcements.
- The use of additives in food-contact materials and articles must adhere to the requirements of GB 9685 and any related public notices.
- Food-contact materials and articles should adhere to the provisions of relevant national food safety standards.
- Identification of compliance requirements under various guidelines including all data requirements.
- Data gap analysis and pre-assessment support
- Technical documentation support
- Pre and post submission support and technical liaison with authorities.
The pesticide regulation in China is called Measures for the Administration of Pesticide Registration. The Measures came into force from the promulgation of the decree of the Ministry of Agriculture and Rural Affairs (MARA) on Aug. 1, 2017. The temporary pesticide registration permit that had been obtained prior to Jun. 1, 2017, would not be renewed; the application for pesticide registration that had not yet been approved would be processed in accordance with the relevant provisions of the Pesticide Management Regulations (PMR).
The PMR aims to better standardize pesticide registration behavior, it is to strengthen pesticide registration management to ensure the safety and effectiveness of pesticides.
Key summaries
- Pesticides produced, traded, and used within the territory of the People's Republic of China shall obtain pesticide registration.
- The registration management of pesticides for overseas use only shall be stipulated separately by MARA.
- The pesticides that have not obtained the pesticide registration permit in accordance with the law shall be treated as fake pesticides.
Responsible unit
- MARA is responsible for the national pesticide registration management and organizing the establishment of a pesticide registration review committee to formulate pesticide registration review rules; the agency responsible for pesticide inspection to which it belongs is responsible for the specific work of national pesticide registration, which is The Institute for the Control of Agrochemicals, Ministry of Agriculture (ICAMA).
- The competent agricultural and rural departments of the provincial people's governments (hereinafter referred to as the provincial agricultural and rural departments) are responsible for accepting applications for pesticide registration within their respective administrative areas, reviewing the application materials, and providing preliminary review opinions. Its subordinate institution, provincial ICAMA, is also responsible for pesticide verification (hereinafter referred to as provincial pesticide verification institutions) assist in the specific work of pesticide registration.
New pesticides: refer to pesticides whose active ingredients have not been approved and registered in China, including new pesticide technical (parent drug) and new pesticide formulations.
Technical medicine: refers to the product obtained in the production process, which is composed of active ingredients and related impurities, and a small amount of additives can be added if necessary.
Parent drug: refers to the product obtained during the production process, which is composed of active ingredients and related impurities, and may contain a small amount of necessary additives and appropriate diluents.
Preparation: refers to the pesticide product in a stable state which is processed from the original pesticide (parent drug) and suitable auxiliary agents, or processed by biological fermentation, plant extraction and other methods.
Auxiliary: refers to any single component or multiple components added to pesticide products, other than active ingredients, that do not have pesticide activity and active ingredient functions, but can or help to improve or improve the physical and chemical properties of pesticide products substance.
The name of the pesticide should use the Chinese generic name of the pesticide or the simplified Chinese generic name, and the name of the botanical pesticide can be represented by the plant name plus the extract. The name of the directly used sanitary pesticide is expressed by the functional description word plus the dosage form.
The content of active ingredients and formulations of pesticides should be set in accordance with the principles of improving quality, protecting the environment, and promoting sustainable agricultural development.
The formulation of the preparation product shall be scientific, reasonable and convenient to use. Single preparation products of the same active ingredient and dosage form, with no more than three content gradients. The active ingredients of the mixed preparation shall not exceed two, and the active ingredients such as herbicides, seed treatment agents and pheromones shall not exceed three. For mixed preparations with the same active ingredients and dosage forms, the proportions shall not exceed three, and the total content gradients of the same proportions shall not exceed three. Pesticides with low active ingredient content for direct use without dilution or dispersion are classified separately.
If the specified adjuvant needs to be added during use, the corresponding test data should be submitted when applying for pesticide registration.
The dilution ratio or concentration of pesticide products should be matched with the application technology.
The specific requirements shall be formulated separately by MARA, and according to the toxicity and hazard of pesticide adjuvants, the list, and limit of prohibited and restricted adjuvants shall be announced and adjusted in a timely manner.
Pesticide manufacturers, companies exporting pesticides to China, or developers of new pesticides will need to apply for pesticide registration in China, depending on their roles there are different requirements apply.
Domestic applicants apply for pesticide registration to the local provincial agricultural and rural departments. Overseas enterprises submit pesticide registration applications to the Ministry of Agriculture and Rural Affairs.
Pesticide production enterprises: refers to domestic enterprises that have obtained pesticide production licenses.
-
Enterprises exporting pesticides to China (hereinafter referred to as overseas enterprises): refers to enterprises that export pesticides produced overseas to China.
-
New pesticide developers: refers to Chinese citizens, legal persons or other organizations that develop new pesticides within the territory of China.
-
For new pesticides jointly developed by multiple entities, one entity should be identified as the applicant, and other cooperative research institutions and relevant test samples should be specified; other subjects may not apply repeatedly.
-
The applicant shall submit the product chemistry, toxicology, efficacy, residues, environmental impact and other test reports, risk assessment reports, labels or instruction sheets, product safety data sheets, relevant documents, application forms, applicant qualification certificates, and materials Authenticity statement and other true, standardized, complete and valid application materials.
The registration test report should be issued by a registration test unit recognized by the MARA, or by a relevant overseas laboratory that has signed a mutual recognition agreement with relevant departments of the Chinese government; experiments and registration experiments of endemic biological species in China should be completed within the territory of China.
When applying for new pesticide registration, the registration application for new pesticide technical and new pesticide formulation shall be submitted at the same time, and pesticide standard products shall be provided.
Within 6 years from the date of new pesticide registration, if other applicants submit the data obtained by themselves or with the authorization and consent of the holder of the new pesticide registration permit to apply for registration, the application for new pesticide registration shall be applied.
MARA or the provincial agricultural and rural department shall deal with the application materials submitted by the applicant according to the respective following circumstances:
- no pesticide registration is required: immediately inform the applicant that it will not be accepted;
- an error occurs in the application information: the applicant is allowed to correct it on the spot;
- the application materials are incomplete or do not conform to the statutory form: the applicant shall be notified on the spot or within 5 working days of all the contents that need to be supplemented and corrected.
- the application materials are complete and conform to the statutory form, or the applicant submits all supplementary materials as required: accepted.
Review and decision will take at least 11 months.
The provincial agriculture and rural departments shall, within 20 working days from the date of accepting the application, conduct a preliminary examination of the materials submitted by the applicant, put forward preliminary examination opinions, and submit them to MARA. If the applicant fails to pass the preliminary examination, the applicant may be notified in writing and explain the reasons as per the applicant's wishes.
After the MARA accepts the application or receives the application materials and preliminary review opinions submitted by the provincial agricultural and rural department, it shall complete the product chemistry, toxicology, efficacy, residue, environmental impact, label proofs, etc. within 9 months and submit the review comments to the Pesticide Registration Review Committee for review.
After receiving the technical review comments, the Pesticide Registration Review Committee will issue review comments in accordance with the pesticide registration review rules. After the application is accepted, the applicant may withdraw the registration application and re-apply after supplementing and improving the relevant information. At the same time, the MARA may also request the applicant to supplement the information according to the opinions of the Pesticide Registration Review Committee.
During the registration review and review period, the types of registration applications submitted by the applicant, as well as the technical requirements and approval procedures they follow, shall not be changed because other applicants have obtained pesticide registration permits during this period. If the new pesticide is approved, the new pesticide registration applications of other applicants that have been accepted can continue to be reviewed and reviewed in accordance with the new pesticide registration approval procedures, or the application can be withdrawn and a new registration application can be submitted.
MARA will make an approval decision within 20 working days from the date of receipt of the review comments. If the requirements are met, the pesticide registration permit will be issued; if the requirements are not met, the applicant will be notified and explain in writing.
The pesticide registration permit is uniformly printed by MARA and the pesticide registration permit is valid for 5 years. If the applicant wish to change the holder of the pesticide registration permit within the validity period of the pesticide registration permit, relevant certification materials should be submitted and application sent to MARA for a replacement of the pesticide registration permit; under any of the following circumstances, the holder of the pesticide registration permit should report Ministry application changes:
- the scope, method or dosage of pesticide use are changed;
- the components other than the active ingredients of pesticides are changed;
- the product toxicity level is changed;
- the content of active ingredients in the original drug product has changed;
- product quality standards are changed;
- there exists any other circumstances prescribed by MARA.
The extension of license will take at least 10 month.
If it is still necessary to continue to produce pesticides or export pesticides to China after the expiration of the validity period, an application for renewal shall be made 90 days before the expiration of the validity period. If the applicant fails to apply for renewal within the time limit, registration must be re-applied. MARA will review the application materials for registration renewal and make a decision on whether to extend the registration before the expiration of the validity period. If there are hidden dangers or risks in safety and effectiveness during the review, it will be submitted to the Pesticide Registration Review Committee for review.
Application for registration change or renewal must be submitted by the holder of the pesticide registration permit to the MARA, with the application form filled in and relevant information submitted. MARA shall complete the review of registration changes within 6 months, form review opinions, submit them to the Pesticide Registration Review Committee for review, and make an approval decision within 20 working days from the date of receipt of the review opinions. If the requirements are met, the registration change will be allowed, and the registration permit number and validity period will remain unchanged; if the requirements are not met, the applicant will be notified in writing and the reasons will be explained.
Provincial agricultural and rural departments should report monitoring and evaluation results to MARA in a timely manner.
- The holder of the pesticide registration permit shall collect and analyze the safety and effectiveness changes of pesticide products, product recalls, and accidents during production and use.
- For pesticide varieties that have been registered for more than 15 years, MARA will organize periodic evaluations based on changes in production use and industrial policies.
- If a registered pesticide is found to have serious harm or greater risk to agriculture, forestry, human and animal safety, agricultural product quality and safety, ecological environment, etc., MARA shall organize a pesticide registration review committee to review and revoke or change the corresponding pesticide registration according to the review results; to decide to disable or restrict the use and make an announcement when necessary.
Supervision and management
In any of the following circumstances, MARA or the provincial agricultural and rural department will not accept the application for pesticide registration; if it has been accepted, it will not be approved:
The authenticity, integrity or normativeness of the application materials does not meet the requirements;
- The applicant does not meet the qualification requirements specified in Article 13 of these Measures;
- The applicant is included in the list of seriously untrustworthy units stipulated by the relevant state departments and restricted from obtaining administrative licenses;
- The pesticides applied for registration belong to the pesticides whose production, operation and use are explicitly prohibited by the relevant state departments, or MARA will no longer register new pesticides according to law;
- The registration test does not meet the provisions of Articles 9 and 10 of the Regulations on the Administration of Pesticide;
- Other circumstances that should not be accepted or approved.
-
- If the applicant conceals relevant information or submits false pesticide registration materials and test samples, its application will not be accepted within 1 year; if the registration has been approved, the pesticide registration permit will be revoked, and its application will not be accepted within 3 years; the pesticide registration permit will be revoked applications will not be accepted within 5 years.
For those who submit false information and test samples, MARA will list the applicant's illegal information in the integrity file and publish it. In any of the following situations, MARA will cancel the pesticide registration permit and announce it:
- the validity period has not been extended;
- the holder of the pesticide registration permit has terminated according to law or is not qualified as a pesticide registration applicant;the pesticide registration information has been transferred according to law;
- other circumstances under which the pesticide registration permit should be cancelled.
Persistent Organic Pollutants (POPs), also referred to as “emerging contaminants”, include a range of chemical substances from diverse applications, ranging from medicines, personal care, household cleaning products, lawn care, agricultural products, among others.
The toxicity of POPs has been long investigated, and researchers found that these chemicals may have long-range mobility and long-lasting duration, leading to environmental contamination and pollution. POPs are often classified as Substance of Very High Concern (SVHC) with hormone disruption consequences, bioaccumulation and biomagnification through the food chain. The presence of POPs in the environment and in human health can lead to several complications, from mild effects to more serious consequences, for example, reproductive disruptions and immunological disorders.
Since POPs are not easily broken down, a characteristic that guarantees its continuation in the environment, the contaminant consequences are still being investigated. In other words, the emerging contaminants are mainly not from new pollutants that recently entered the environment. Instead, there is a good understanding of contaminants, but the consequences of their exposure are still being discovered.
Countries have created a set of rules and regulations to regulate, control, and mitigate POPs use and its effects on the environment and on human health. The essence of these legislations is to eliminate the production, trading, and use of such chemicals, including storage and waste management.
International effort in addressing POPs starts in the late 1990s. The initial protocol to address POPs was implemented in 1998, signing 16 chemicals to phase out according to previously established risk criteria. The Aarhus Protocol also impose parties to reduce their emissions below 1990 levels.
The Stockholm Convention is an international conference specifically for POPs proposed in May 2001 in Sweden (complementing the Aarhus Protocol), entering into force in 2004. Among the objectives, the Stockholm Convention aims to:
- Prohibit, eliminate, and restrict several POPs in the production, use, import, and export.
- Ensure that waste containing POPs is managed safely and in an environmentally sound manner.
- Promote tools for information exchange, public access, awareness and education, research, development and monitoring, reporting, and implementation of plans to fight POPs pollution.
The Stockholm Convention is considered the main international instrument to address POPs. Most countries adhere to the Stockholm Convention.
China signed the Stockholm Convention on its launch in 2001 and ratified the Convention in 2004. Since then, the initial list of 12 POPs listed in the Stockholm Convention entered the government agenda. For example, the Regulation for Pesticide Management, issued by the State Council in 1997 and amended in 2002, is a comprehensive law that strengthened the import and export, registration, production, utilization, management, and transportation of pesticides, especially those of POPs. The Regulation for Pesticide Management forbids enterprises or individuals to produce, utilize, or trade products such as Hexachlorobenzene (HCB), Bexachloridge (HCH), and Dichlorodiphenyltrichloroethane (DDT).
In 2007, the Chinese government issued the National Implementation Plan for the Stockholm Convention on Persistent Organic Pollutants, dividing the implementation goals by stages (2010, 2010-2015, and after 2015), by region, and type of industry. The overall objective, as expected, is to reduce or eliminate the environmental and health risks posed by the POPs through the effective implementation of several action plans.
- By 2010
- Eliminate the production, use, import, and export of certain pesticide POPs
- Control of PCBs used in PCBs-containing equipment in use
- Reduce or eliminate releases of unintentionally produced POPs
- Reduce or eliminate releases of POPs from stockpiles and wastes
- By 2015
- Eliminate the use of PCBs in currently used equipment containing PCBs
- Reduce or eliminate releases of unintentionally produced POPs
- Reduce or eliminate releases originating from POPs stockpiles and wastes
- Manage POPs contaminated sites
- After 2015 (long-term aims)
- Complete the identification of currently used equipment containing PCBs and eliminate uses of PCBs by 2025
- Promote the Best Available Techniques and Best Environmental Practices in all relevant areas for maximum reduction of Dioxin releases
- Improve the inventories of POPs wastes and contaminated sites, pursuing their gradual recovery
The overall steps for the National Implementation Plan for POPs are depicted below:
Currently, China’s legislation addresses phasing-out, reduction, control, and disposal of POPs or contaminated articles with POPs, specifically with alpha hexachlorocyclohexan, beta hexachlorocyclohexane, chlordecone, hexabromobiphenyl, hexabromodiphenyl ether, heptabromodiphenyl ether, lindane, pentachlorobenzene, perfluorooctane sulfonic acid, tetrabromodiphenyl ether, pentabromodiphenyl ether, endosulfan, and hexabromocyclododecane.

 Twitter
Twitter
