GPC Newsletter Oct-2020
Toys, both imported and locally manufactured, constitute a significant market for many countries. Over the past decides, many countries have adopted toy safety regulations or reinforced their current regulatory framework. The United States adopted the Consumer Product Safety Improvement Act (CPSIA) in 2008, and the European Union adopted Directive 2009/48/EC on the Safety of Toys in 2009. They were soon followed by the Eurasian Economic Union, which adopted regulation CU TR 008/2011 in 2011. Turkey adopted a regulation based on the EU Directive on Toy Safety in 2016, and India’s Toy Quality Control Order was enacted in early 2020.
In countries where comprehensive toy safety regulations have been adopted, toys are most often subject to a two-tier regulatory framework. First, a legislative act sets out the general obligations placed upon the industry. Public authorities then request an authorised standardisation body to draft detailed safety requirements in the form of standards. For example, the European Union, the United States, and the Eurasian Economic Union all have both a regulation and a set of safety standards.
Regulatory frameworks applicable to toy safety may vary from one jurisdiction to another. They tend, however, to share some common features. Such features typically include a requirement to comply with safety provisions in relation to the toys’ physical, mechanical, electrical, radioactive, flammability-related and, of course, chemical properties. The latter generally include lists of substances which may not be contained in toys, substances which may be contained under certain concentrations, and migration limits.
Other common features often consist in a requirement for manufacturers to affix warnings, ensure the toys’ traceability, and perform a conformity assessment prior to placing the toys on the market. Conformity assessment may be performed according to various modalities (e.g. self-assessment or third-party assessment) and normally results in the issuance of a declaration of conformity.
Regulatory News
Canada : Canadian Government plans order adding ‘plastic manufactured items’ to Schedule One of Cepa
Canada is one of the countries that take action on plastic waste and pollution. On October 10th 2020 the Canadian government plans to propose an order to add "plastic manufactured items" to Schedule One of the Canadian Environmental Protection Act (Cepa). Schedule One is the country’s list of toxic substances.
Through listing substance in Schedule One it will give the government a tool to address plastic pollution at different stages of the lifecycle of plastic manufactured items, such as manufacture, import, sale, use and disposal of articles.
The aim is to set up regulatory actions to address plastic pollution and is part of the government’s broader plan to manage plastic products, recover and recycle plastics and reach zero plastic waste by 2030.
The government also laid out plans to ban six specific types of single use plastics:
- checkout bags;
- straws;
- stir sticks;
- six-pack rings;
- cutlery; and
- foodware made from hard-to-recycle plastics.
The government also released its final scientific assessment of plastic pollution, which concluded "action is needed to reduce macro-plastics and microplastics that end up in the environment". The assessment focused on plastic waste entering the environment and did not review the efficacy of waste management processes like mechanical or chemical recycling.
Environment and Climate Change Canada said it will accept comments until 9 December on its plan to ban the six categories of single-use products. Regulations would then be finalised by the end of 2021.
On October 14 the European Commission published the comprehensive Chemical strategy (EU Chemicals Strategy for Sustainability). The Strategy is the first step toward a zero pollution ambition outlined in the European Green Deal. The strategy covers a variety of dimensions, including non-toxic material cycles, banning the most harmful chemicals in consumer products, phasing out the use of per- and polyfluoroalkyl substances (PFAS) in the EU, establishing a simpler “one substance one assessment” process for the risk and hazard assessment of chemicals.
The Strategy is expected to boost innovation for safe and sustainable chemicals and increase protection of human health and the environment against hazardous chemicals.
On 17 March 2020 the Eu Commission notified the WTO Committee on TBT following the publication of a draft Directive amending Directive 2009/48/EC in the following manner:
In paragraph 3 of point 11 of Part III of Annex 2 of Directive 2009/48/EC (list of allergenic fragrances the name of which shall be listed on the toy, on an affixed label, on the packaging or in an accompanying leaflet if added to a toy at concentrations exceeding 100mg/kg):
The following entry is modified:
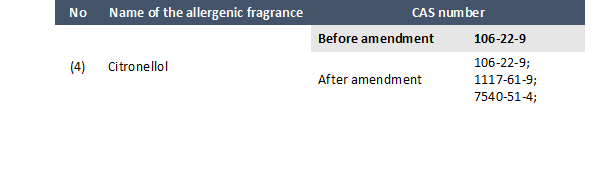
Click here to see the entries that are added.
On 17 March 2020 the EU Commission notified the WTO Committee on TBT following the publication of a draft Directive amending Directive 2009/48/EC in the following manner:
- In paragraph 1 of point 11 of Part III of Annex 2 of Directive 2009/48/EC (list of allergenic fragrances which shall not be contained in toys) the following entries are added:
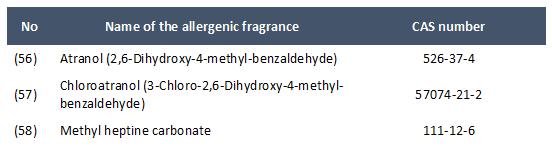
On 8 September 2020 the EU Commission notified the WTO Committee on TBT following the publication of a draft Directive amending Directive 2009/48/EC as regards specific limit values for aniline in toys.
In Appendix C to Annex II to Directive 2009/48/EC (Specific limit values for chemicals used in toys intended for use by children under 36 months or in other toys intended to be placed in the mouth) the following entry is added:

On 14 September 2020, Denmark notified the WTO Committee on Technical Barriers to Trade of its enactment of an Executive order on safety requirement for toys. The purpose of the executive order is to transpose into Danish law Directive 2019/1922/UE modifying migration limits for aluminium and Directive 2019/1929/UE laying out limit values for formaldehyde.
The two aforementioned Directives amend Directive 2009/48 on the safety of toys in the following manner:
- In point 13 of part III of Annex II to Directive 2009/48/EC (migration limits for toys and components not to be exceeded):
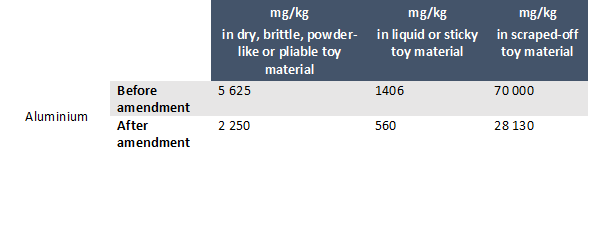
- In Appendix C to Annex II to Directive 2009/48/EC (Specific limit values for chemicals used in toys intended for use by children under 36 months or in other toys intended to be placed in the mouth) the following entry is added:
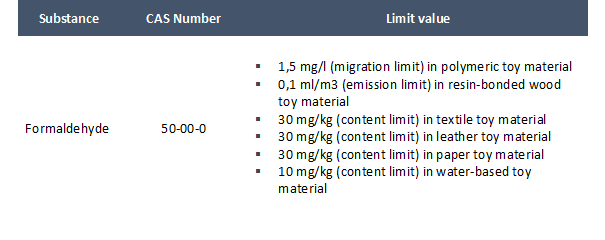
EU Member States are required to transpose Directive 2019/1922 by 19 May 2021 and Directive 2019/1929 by 20 May 2021.
With Brexit being implemented from 31st January 2020, the UK has formally left the EU with a withdrawal deal and it is currently into an 11-month transition period. The UK is no longer a member state of the EU and until the end of the transition period market access would continue the same terms as before. During this transition period, the UK will continue to remain strongly committed to the effective and safe management of chemicals. The UK will exit the transition period on the already set date 31st December 2020.
The formal, ninth and final round of talks on a post-Brexit free trade treaty between the EU and the UK which had begun on September 29 were called off early in this month due to lack of progress. The UK and Europe are however set to resume the trade talks later this week.
At the end of the Transition Period, the EU REACH Regulation will be brought into UK law under the European Union (Withdrawal) Act 2018 and will be known as UK REACH regulation. REACH, and related legislation, will be replicated in the UK with the necessary changes to make it functional from 1st January 2021. The key principles of the EU REACH Regulation will be retained in UK REACH.
The Health and Safety Executive (HSE) will play a key role in the UK's chemicals regulatory process together with the Department for Environment, Food and Rural Affairs (Defra) and the Environment Agency (EA) to ensure the effective and safe management of chemicals to safeguard human health and the environment.
The Department for Environment, Food and Rural Affairs (Defra), early in September 2020, had announced tonnage band specific, staggered submission deadlines that will apply for the full submission of data. The full registration dossiers would hence need to be submitted within 2, 4 or 6 years, starting from October 28, 2021.
In a private and very recent (October 2020) communication of Global Product Compliance (Europe) AB with the Health and Safety Executive (HSE), they have indicated that, the chemicals legislation that will apply in the UK after the end of the transition period (31st December 2020) is subject to ongoing EU-UK trade negotiations, and the precise details are still being decided. Having said that, the legislation is likely to be very similar to that which was drawn up for a “no deal” scenario. The HSE authorities have indicated that the future legislation is likely to be similar to that which was drawn up for a “no deal” scenario (UK REACH) and as specified in the original ‘no-deal’ Statutory Instrument for UK REACH. The SI will be amended to account for any changes, including the date in which it comes into force any changes to REACH that can be retained as EU law. The notification/registration processes for the UK and non-UK based companies under UK REACH will be as per the most recent government guidance as published on 1st September 2020. The authorities have indicated that, UK REACH will apply to UK companies and non-UK companies will not have any duties under UK REACH. However, a non-UK manufacturer, formulator or producer of articles will have to appoint a UK-based only representative (OR) to fulfil the obligations of the UK importers.
Global Product Compliance (Europe) AB has already set up a UK based OR entity ‘GPC UK’, to support its existing and new potential clients, to be able to comply with the challenges posed because of Brexit on substance exports to the UK.
For more information or queries, please write to us at compliance@uk.gpcregulatory.com
GPC invites you to our webinar on UK REACH regulations and its impacts for manufacturers of cosmetic products and raw materials on Nov. 6th at 14:00 (CEST)/18:30 (IST). Register here.
.png)
In September 2020, the Ukrainian government published a draft resolution of changes to the Ukrainian Technical regulation on Toy’s safety.
The changes in the Technical Regulation are prepared to bring the provisions of the Technical Regulation in line with the provisions of Directive 2009/48/EC of the European Parliament and of the Council which also provides the basis for the Ukrainian Technical Regulation on Toy’s Safety. The proposed changes to the regulation suggest for lowering the limits for chromium (VI) and aluminium (table 1) in toys and toy materials, as well as limiting the amount of formaldehyde (table 2).
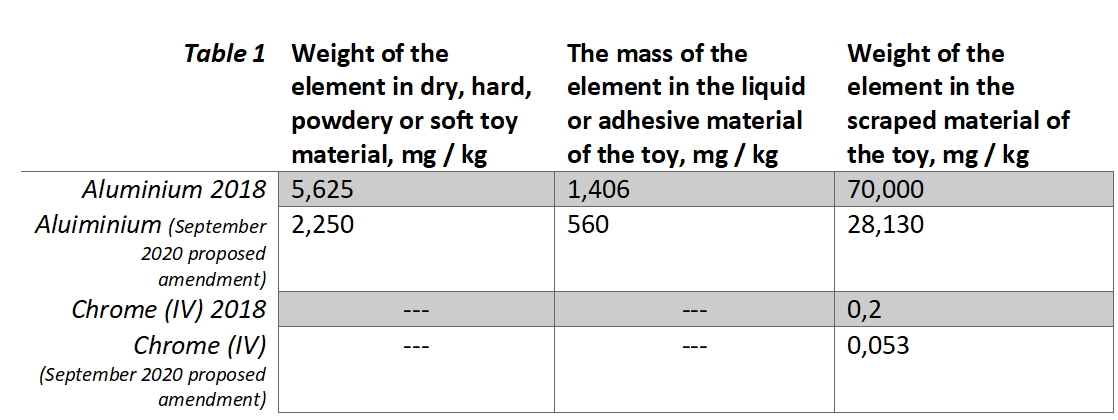
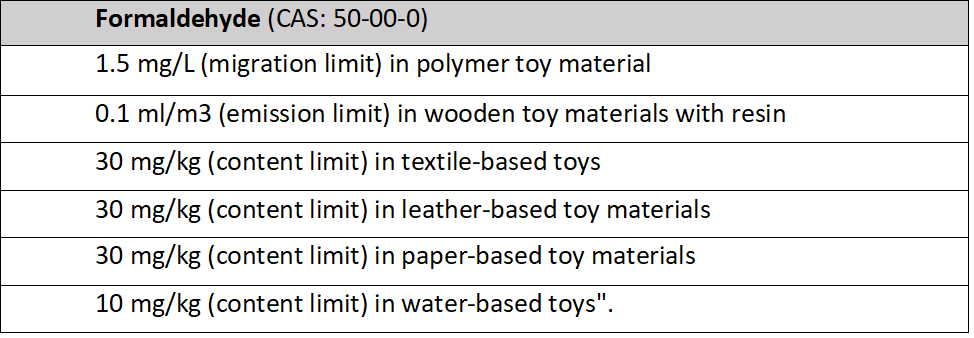
Table 2
According to the Ukrainian Cabinet of Ministers, the implementation of the law will have a positive impact since it demands more stringent requirements for limitation of chemicals that are hazardous and/or are classified as carcinogenic, mutated or toxic. Consequently, this resolution will ensure a higher quality level of toys and better protection for consumers.
Ukraine’s new Technical Regulation on Toy’s Safety was adopted in February 2018 and replaced the former Technical Regulation adopted in 2013. However, all toys that complied with the 2013 Technical Regulation were still allowed for circulation on the Ukrainian market even after the new Technical Regulations entrance into force. The institution responsible in ensuring the implementation of the Technical Regulation on Toy’s Safety is the Ministry for Development of Economy, Trade and Agriculture of Ukraine.
Owing our experience and growth to the requests and guidance of our client base, we, Global Product Compliance have established our branch in Turkey on February 2020, as already known.
Since then, we have been working hard to help our loyal client base with their Turkish businesses, and prove their trust which we reciprocally built past years in many other regulations around the globe.
By the end of October, we are proudly announcing that we have already surpassed 1000 complete & ongoing pre-registrations under KKDIK (Turkey-REACH), securing the bussiness of our new and existing clients in Turkey for the upcoming years. Many thanks for your interests and trust in us!
If you haven't pre-registered your substances yet, send us your substances and as your seamless extension in global regulatory compliance, GPC will swiftly help you securing your interest in the Turkish market. Please note that the deadline for pre-registration is 31st December 2020, less then 60 days!
The Turkish Toy Regulation has entered force on October 4th, 2016 and has been regulating the toy products since then with the purpose of defining methods and foundations of security and free circulation of toys.
The regulation was updated recently in December 2019 to amend some maximum limits of substance containment such as chromium and lead, as well as Overall Migration Limits (OML) of Bisphenol A and Phenol in the light of recent data to ensure toy security.
Limit of Chromium (VI) in recyclable toy products has been lowered from 0.2 mg/kg to 0.053 mg/kg, while the presence of lead was strictly dropped to 2 mg/kg from 13.5 mg/kg in breakable or soft toys, from 3.4 mg/kg to 0.5 for sticky or fluid toys, and finally from 160 to only 23 mg/kg for recyclable toys.
See more on the amendment on Toy Regulation in Turkey.
The Union Cabinet ratified ban on seven Persistent Organic Pollutants (POP) listed under Stockholm Convention.
The Stockholm Convention is a global treaty to protect human health and environment from POPs, which are identified chemical substances that persist in the environment, bio-accumulate in living organisms, adversely affect human health/ environment and have the property of long-range environmental transport (LRET).
The Cabinet’s approval for ratification of POPs demonstrates India’s commitment to meet its international obligations with regard to protection of environment and human health.
Safety of Toys has been brought under compulsory BIS Certification as per Toys ((Quality Control) Order from 1 September 2020. The order was notified to the WTO in February this year. For BIS Certification, toys have classified into two categories: (1) Non-Electric Toys and (2) Electric Toys.
The chemicals and the standards with which they must conform are:
- Acrylonitrile – used in the production of plastics (IS 12540:1988);
- Maleic anhydride – used in the production of plastics (IS 5149: 2020);
- Methyl acrylate – used to manufacture carpet fibres (IS 14707: 1999);
- Ethyl acrylate – a precursor to polymers and other monomers (IS 14709:1999);
- N-butyl acrylate – used in paints, coatings, adhesives, textiles and plastics (IS 14709:1999); and
- Styrene (vinyl benzene) – used as a precursor in the manufacture of polystyrene (IS 4105:2020).
Products containing any of these chemicals will require certification to prove conformity, and companies will need to display the BIS mark (Bureau of Indian Standards) on all packaging.
South Korean government published a list of 115 chemical substances where a simplified approval process is applicable. These 115 substances are active biocidal substances that were already approved under the EU BPR (Biocidal Product Regulation) and the US Fifra (Federal Insecticide, Fungicide, and Rodenticide Act). They are used as disinfectants, algicides, repellents, rodenticides, and insecticides (Product group 1).
Although a substance is on the list, if a substance is considered to be high risk and toxic, or there are risks the approval is not renewed under the EU BPR then the South Korean government will not approve the substance. Of these 115 substances, ten substances have the possibility of non-approval:
- formaldehyde; formalin for use as a disinfectant;
- coumatetralyl for use as a rodenticide;
- boric acid for use as a disinfectant or insecticide;
- polyhexamethylene biguanide hydrochloride; PHMB for use as a disinfectant;
- brodifacoum; 4-hydroxy-3-(3-(4'-bromo-4-biphenylyl)-1,2,3,4-tetrahydro-1-naphthyl)coumarin for use as a rodenticide; and
- flocoumafen for use as rodenticides or insecticide.
By 16 October 2020, applicants are required to submit a substance identity report (SID), product, and exposure information. Even after submitting this information to the government, applicants must submit full dossiers by December 2022. The dossiers include SID reports that are issued by an ISO/IEC 17025 accredited body, less than three-years-old, and produced with three batches in triplicate. It is emphasized that most EU data are more than three-years-old since they are produced, so applicants shall review and generate new SID reports accordingly.
To get K-BPR approvals, applicants should analyze specific data requirements under K-BPR, which might be different from the EU or US dossiers. For instance, in the case of bioaccumulation study on fish, the bioconcentration factor (BCF) value in the EU is above 2,000 whereas in South Korea, 500.
Affected stakeholders and implications
Those who manufacture or import biocidal active substances should submit approval dossiers on time. The grace period of the first product group (Disinfectants, algicide, repellents, rodenticides, and insecticides) ends by December 2022. It is recommended to submit all dossiers by August 2021, considering the time taken for the dossier evaluation. The South Korean government will publish a list of additional test data that has to be submitted in June 2021.
Last update: 10/22/2020
South Korea restricts the contents of Heavy metals, Phthalates, and Nitrosamines in children’s products since 4 June 2020. As a result, manufacturer and importer of children’s products should test products to ensure not to exceed the maximum thresholds.
Children’s Products Safety Special Act is to limit the contents of hazardous substances in children’s products such as toys and textiles intended to be used by children aged under 13. The Ministry of Trade, Industry, and Energy set out subordinate regulations along with the Act since 2017.
Heavy metals contents in paints, coatings, and paper should not exceed the maximum limits as follows.
- antimony – 60mg/kg;
- arsenic – 25mg/kg;
- barium – 1,000mg/kg;
- cadmium – 75mg/kg;
- chromium – 60mg/kg;
- lead – 90mg/kg;
- mercury – 60mg/kg; and
- selenium – 500mg/kg.
The total contents of lead and cadmium shall not be more than 100mg/kg and 75mg/kg, respectively.
The phthalates including DEHP (Di-(2-ethylhexyl) phthalate), DBP (Dibutyl phthalate), BBP (Benzyl butyl phthalate), DINP (Diisononyl phthalate), DIDP (Diisodecyl phthalate), DnOP (Di-n-octyl phthalate) should not exceed 0.1%.
For oral products used by infants aged under 36 months, such as dummies and toothbrushes, Nitrosamines, and potential substances that can produce Nitrosamines – which can increase the risk of cancer- should not be more than 0.01mg/kg.
Formaldehyde in textiles contacting with skin should not exceed 75mg/kg. Aromatic amines are limited to 30mg/kg, which is applied to dyed textiles. The pH of all products should be in the range of 4.0-7.5.
Affected stakeholders and implications
Children’s products manufacturer and importer should comply with a new standard by testing their products. The contents of Heavy metals, Phthalates, and Nitrosamines in children’s products are restricted.
Last update: 10/22/2020
Australia issued new legislation for toys containing magnets,” Consumer Goods (Toys Containing Magnets) Safety Standard 2020.” This new legislation will repeal the existing “Consumer Product Safety Standard for Children’s Toys Containing Magnets (Consumer Protection Notice No. 5 of 2010) (Federal Register of Legislation No. F2010L00195). Any children’s toys containing magnets must comply with this new requirement from August 28, 2021.
This law will be applicable to all magnetic toys designed for use in play by a child under 14 years of age, supplied with one or more magnets or magnetic components.
The new law mandates to comply with the following clauses from any one of the instruments mentioned in the below table.
|
Countries |
Instruments |
Clauses |
|
Australia / New Zealand Standard |
AS/NZS ISO 8124.1:2019 Safety of toys Part 1: Safety aspects related to mechanical and physical properties |
4.1, and 4.31 |
|
European Standard |
EN 71‑1:2014+A1:2018: Safety of toys ‑ Part 1: Mechanical and physical properties |
4.23 |
|
International Standard |
ISO 8124‑1:2018 Safety of toys —Part 1: Safety aspects related to mechanical and physical properties |
4.1, and 4.31 |
|
US Standard |
American Society for Testing and Materials Standard ASTM F963 ‑ 17 Standard Consumer Safety Specification for Toy Safety |
4.38, 8.5 and 8.9 |
During the transition period till 27 august 2021, magnetic toy must comply with either any one of the following section requirements, Australian/New Zealand Standard (section 9), European Standard (section 10), International Standard (section 11), US Standard (section 12) or the Consumer Product Safety Standard for Children’s Toys Containing Magnets (Consumer Protection Notice No. 5 of 2010).
However, this new law is not applicable to sporting goods, camping goods, bicycles, home and public playground equipment, trampolines, electronic game units, models powered by combustion or steam engines, and fashion jewelry.
Vietnam has reopened the nomination of chemicals for inclusion in its draft national inventory until 15 April next year.
In the first round of nominations, the Vietnamese Centre for Emergency Response to Chemicals (VCERC) accepted submissions from industry from early April until 30 May this year, after which it would begin checking and verifying the nominated substances. It was the final opportunity for industry to nominate substances.
After May’2020 several industry bodies raised concerns previously about the lack of time to gather the required information and also because of the world pandemic situation, so this time it has given a longer period of six months to make submissions.
Authorities expects to complete and publish the national chemical inventory in 2021 or 2022.
After the inventory is published, any substance which is not listed on it or not officially recognised by Vietnam, will be considered as a new chemical and subject to risk assessment. More clarity is expected on how new chemicals will be regulated by the authorities.
Following information needs to be submitted for doing the nomination on Vietnamese chemical inventory and it has to be done by the Vietnamese legal entity
-
chemical name;
-
Cas number;
-
safety data sheets (SDSs); and
-
evidence or documentation proving the chemical has been used in the Vietnamese market (this can include purchasing contracts and invoices).
For more details please contact us at compliance@gpcregualtory.com
Some chemicals are source of increasing concern as they can cause allergic reactions after skin contact. In view of the likely exposure to sensitising chemicals found in textiles, leather, hide and fur articles, the Swedish Chemicals Agency (KemI) and the French Agency for Food, Environmental and Occupational Health and Safety (ANSES) analysed several risk management options and proposed a restriction under REACH as the most appropriate measure to manage the possible risks that such chemicals pose to citizens. The Committee for Socio-economic Analysis (SEAC) also supports France and Sweden’s proposal to limit the use of skin sensitising substances in clothing, footwear and other articles with similar skin contact.
KemI and ANSES’s proposal pursues to limit the concentrations of substances that have a harmonised classification as skin sensitisers in Categories 1/1A/1B listed in the CLP Regulation and that are present in these articles. It also covers disperse dyes which may cause allergic skin reactions – even if the dyes do not have a harmonised classification as skin sensitisers.
The proposal introduces a link with the CLP Regulation meaning that any substance that is classified as a skin sensitiser in the future under CLP would automatically be covered by the restriction. When substances are automatically added to the restriction, SEAC recommends a transitional period of three years between classification and the conditions of the restriction taking effect to allow manufacturers to adapt.
SEAC adopted its final opinion on France and Sweden’s proposal to restrict skin sensitising substances in textile, leather, synthetic leather, hide and fur articles, that are placed on the market for the first time. This further follows an earlier opinion by the Committee for Risk Assessment (RAC) in March 2020 and both committees concluded that an EU-wide restriction is the most appropriate means to address the risks to EU citizens.
In their September meetings, RAC and SEAC also discussed applications for authorisation, other restriction proposals and harmonised classification and labelling which included following conclusions.
- SEAC agreed its final opinion on ECHA’s proposal to restrict the placing on the market of calcium cyanamide used as a fertiliser.
- SEAC adopted its final opinion on ECHA’s proposal to restrict five cobalt salts i.e. Cobalt carbonate, Cobalt diacetate, Cobalt dinitrate, Cobalt sulphate and Cobalt dichloride. As all of them are classified as carcinogenic, mutagenic and reprotoxic (CMR).
- SEAC accepted its final opinion on ECHA’s proposal to restrict formaldehyde in articles, road vehicles and aircraft.
- SEAC also received an update on the consultation comments received on ECHA’s proposed restriction of intentionally-added microplastics. The 60-day consultation on SEAC’s draft opinion ended on 1 September 2020, with 211 comments received. SEAC is expected to adopt its final opinion in December 2020.

 Twitter
Twitter
